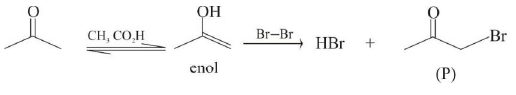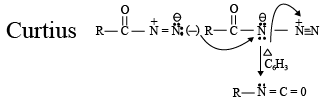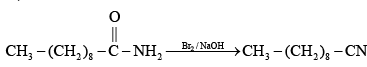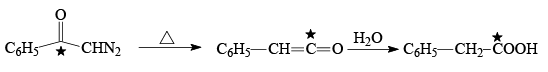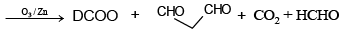JEE Advanced (One or More Correct Option): Aldehydes, Ketones & Carboxylic Acids | Chapter-wise Tests for JEE Main & Advanced PDF Download
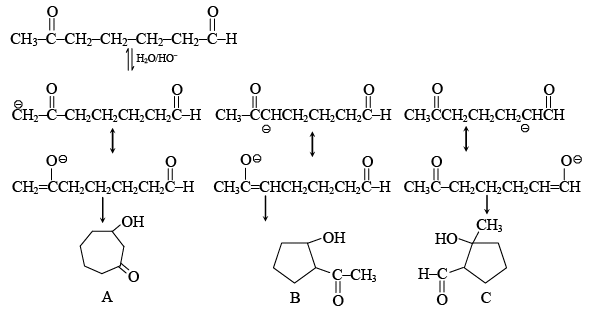
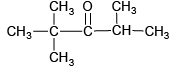
Q.1. When 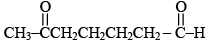 undergoes aldol addition reaction, then
undergoes aldol addition reaction, then
(a) the total number of products formed is 3 and 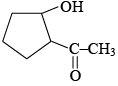 is the major product
is the major product
(b) The total number of products formed is 2 and the major product is 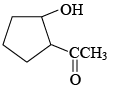
(c) The total number of products formed is 2 and 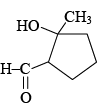 is the major product
is the major product
(d) The total number of products formed is 3 and 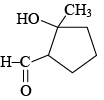 is the major product.
is the major product.
Correct Answer is option (a)
five membered ring is formed in preference to seven membered ring. B is formed more because double bond of the enolate leading to B is more substituted making enolate more stable.
Q.2. Which of the following statement(s) is/are correct?
(a) When D-galactose is oxidised by HNO3, it gives a meso isomer.
(b) Aldoses react with Fehling's solution and PhNHNH2 but not with NaHSO3.
(c) The smallest aldose to form a cyclic hemi-acetal must have 4 carbon atoms.
(d) D-glucose and D-fructose can be differentiated using Benedict's solution.
Correct Answer is option (a, b, d)
Aldose does not react with NaHSO3 because of cyclic structure of aldose.
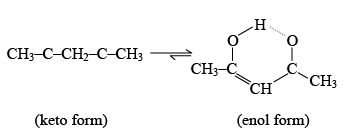
Q.3. Consider the following statements
(1) 2, 4-dicarbonyl compounds such as, 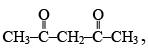 are mostly enolised
are mostly enolised
(2) The compound (a) given below exists mainly in enol form (almost 100%)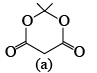
(3) The given below compound (b) exists only in keto form 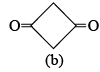 of these statements:
of these statements:
(a) statement (1) is correct.
(b) statements (2) and (3) are correct.
(c) statement (1) is correct but statement (2) is wrong.
(d) all statements are incorrect.
Correct Answer is option (a, b)
Statement (1), (2) and (3) are correct 2, 4-Pentaredione usually exists in enol form because of cross-conjugation and intramolecular H-bonding of form.
Similarly, compound (a) is almost totally enolised because enol form is stabilized by delocalization.
Compound (b) on enolisation will convert into antiaromatic compound which is highly unstable and thus it exists only in keto form.
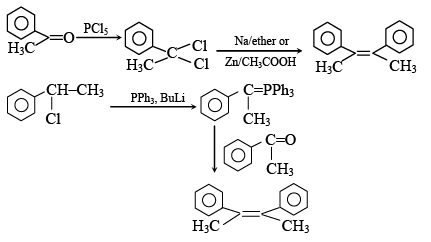
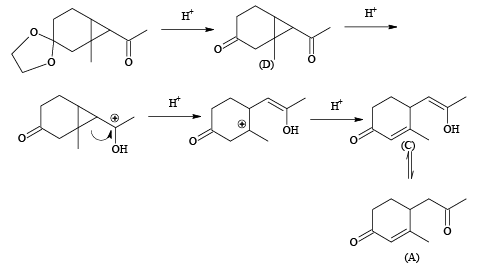
Q.4.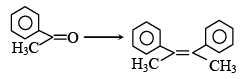
The above conversion can be done successfully with
(a) (I) PCl5; (II) Zn, CH3COOH
(b) 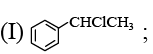 (II) PPh3 ; (III) BuLi
(II) PPh3 ; (III) BuLi
(c) (I) PCl5; (II) Na, ether
(d) (I) LiAlH4 (II) SOCl2 (III) Na, ether
Correct Answer is option (a, b, c)
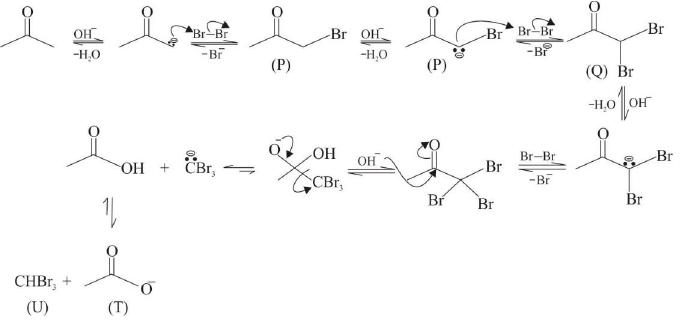
Q.5. After completion of the reactions (I & II), the organic compound(s) in the reaction mixture is/are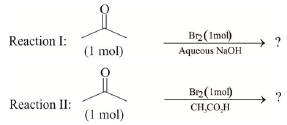
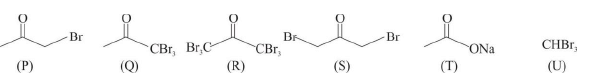
The product(s) is/are among the following compounds.
(a) Reaction I: (U) & (T)
(b) Reaction II: (P)
(c) Reaction I: Acetone, (U) & (T)
(d) Reaction II: (Q)
Correct Answer is option (b, c)
Reaction I :
Reaction II:
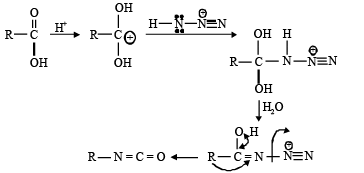
Q.6. Curtius reaction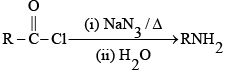
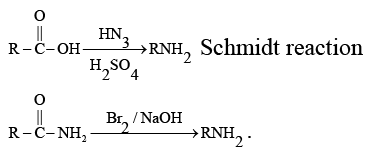
What are common in above reaction?
(a) intermediate is R-N=C=O
(b) No Nitrene formation
(c) All the reactions involve intra molecular migration of alkyl group.
(d) Stereo chemistry of R group is maintained and here migration of R from carbon to Nitrogen takes place
Correct Answer is option (a, b, c, d)
No evidence for formation of Nitrene
Migration is intra molecular no internee formation.
Q.7. Which can give white crystalline product with NaHSO3
(a) 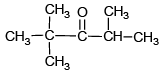
(b) 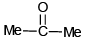
(c) 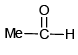
(d) 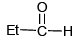
Correct Answer is option (b, c, d)
Higher the hindrance lower will be the reaction of carbonyl compound with NaHSO3. due to weak nucleophilic nature of NaHSO3 it will not react with HCl
Q.8. Which of the following reactions is/are correctly indicating the formation of major product?
(a) 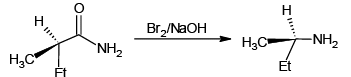
(b) 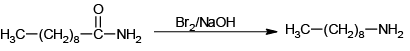
(c) 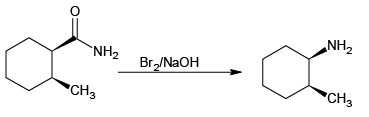
(d) 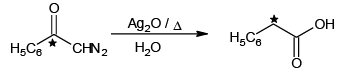
Correct Answer is option (a, c)
(b)
Hoffmann reaction is not given by chain containing seven or more carbons. They give nitrites.
(d)
Q.9. Identify the possible products formed during the following reaction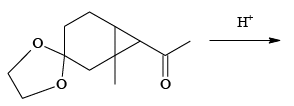
(a) 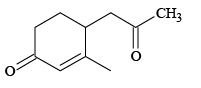
(b) 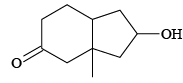
(c) 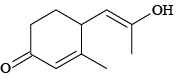
(d) 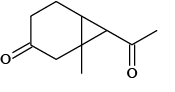
Correct Answer is option (a, c, d)
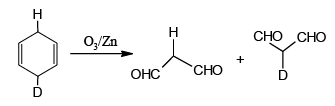
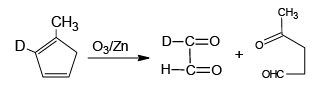
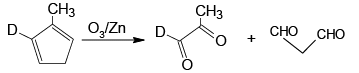
Q.10. Which of the following isomer of C6H8 shall give OHC – CH2 – CHO on ozonolysis in presence of Zn
(a) 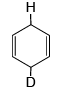
(b) 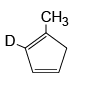
(c) 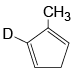
(d) CD2 = CH – CH2 – CH = C = CH2
Correct Answer is option (a, c, d)
(a)
(b)
(c)
(d) D2 = CH – CH2 – CH = C = CH2
|
446 docs|929 tests
|