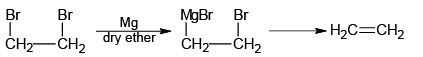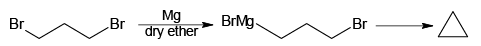JEE Advanced (One or More Correct Option): Haloalkanes & Haloarenes | Chapter-wise Tests for JEE Main & Advanced PDF Download
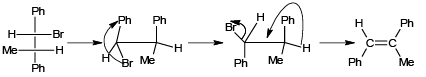
Q.1. Which of the following reactions is/are correct?
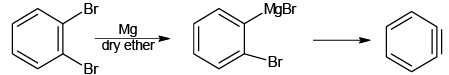
(a) 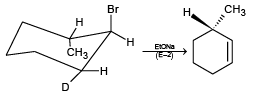
(b) 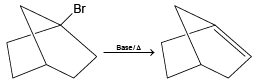
(c) 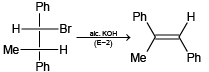
(d) 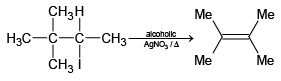
Correct Answer is option is (a, c, d)
(a) In the given configuration, Br and D are in the antiperiplanar position and hence product is correct.
(b) (C = C) bond cannot be formed at bridge head position.
(c)
(d) It is E1 reaction, so product is correct.
Hence a, c, d is correct.
Q.2.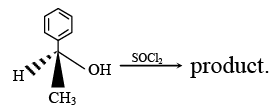 Which is/are incorrect statement/s regarding the above reaction?
Which is/are incorrect statement/s regarding the above reaction?
(a) Kinetically reaction is unimolecular.
(b) Stereo chemically the configuration is retained.
(c) Stereo chemically Walden inversion takes place.
(d) Addition of pyridine drives the configuration from retention to Walden inversion.
Correct Answer is option is (a, c)
Rate = Substrate][Nu-]
So it is bimolecular
Due to internal attack of Cl in 2nd step, configuration is retained.
Addition of pyridine produces freeand in presence of strong nucleophile back side attack is more preferred. Hence inversion takes place.
Q.3. In which of the following cases rate is dependent upon concentration of nucleophile?
(a) 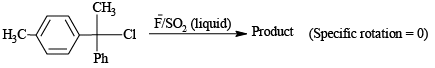
(b) 
(c) 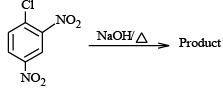
(d) 
Correct Answer is option is (b, c)
(b) is reversible reaction therefore increasing concentration of (Nu-) reaction proceed in forward direction.
(c) is aromatic nuleophilic substitution (SNAr ) and will depend on concentration of nucleophile
Q.4. The intermediate(s) formed during the reaction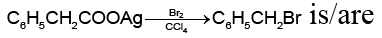
(a) 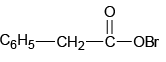
(b) 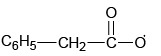
(c) 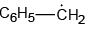
(d) 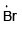
Correct Answer is option is (a, b, c, d)
Q.5. Which of the following will not form Grignard reagent with Mg/dry ether?
(a) 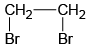
(b) 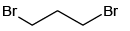
(c) 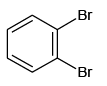
(d) 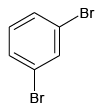
Correct Answer is option is (a, b, c)
(a)
(b)
(c)
Q.6. Which of the following statements are true for the SN1 reactions of alkyl halides?
(a) The rate of SN1 reaction depends on the concentration of the alkyl halide
(b) The SN1 reactions of alkyl halides are favoured by polar solvents.
(c) The rate of an SN1 reaction depends on the concentration of the nucleophile
(d) The rate of an SN1 reaction depends on the stability of carbocation produced from alkyl halide.
Correct Answer is option is (a, b, d)
SN1 is unimolecular and it follows first order kinetics and the rate of reaction does not depend upon concentration of the nucleophile.
Q.7. Me2CH-CH2-Br 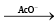 product if instead of acetate ion ethoxide ion is used as base under same reaction conditions then the correct fact regarding products are
product if instead of acetate ion ethoxide ion is used as base under same reaction conditions then the correct fact regarding products are
(a) Me2CH CH2 OC2H5 is obtained as main product.
(b) Reaction acquires SN2 route.
(c) Me3COC2H5 is formed as minor product.
(d) (D) Me2C = CH2 is formed as main product.
Correct Answer is option is (a, b)
Acetate ion is good nucleophile rather base so substitution is the main reaction
Q.8. Under SN1 conditions, the hydrolysis of neopentyl bromide, 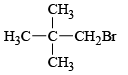 gives
gives
(a) 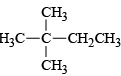
(b) 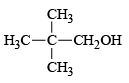
(c) 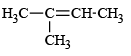
(d) None of these
Correct Answer is option is (b, c)
Under SN1 conditions, carbocation is formed due to which rearrangement also takes place.
Q.9. Mark the correct choice (s)
(a) 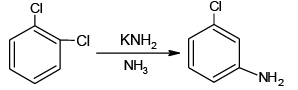
(b) 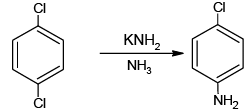
(c) 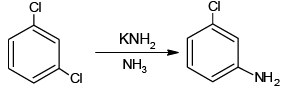
(d) 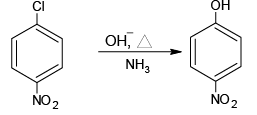
Correct Answer is option is (a, b, c, d)
All the four are correct choices. The first three reactions involve benzyne intermediate.
Q.10. The correct statements among the following statements are
(a) In SN1 reaction both inversion and retention of configuration takes place.
(b) In SN2 reaction retention of configuration takes place
(c) SN1 reaction involves the formation of carbocation intermediate.
(d) In SN1 reaction the order of reactivity is 1º > 2º > 3º
Correct Answer is option is (a, c)
(b) is incorrect because in SN2 reaction inversion of configuration takes place
(d) is incorrect because the order of reactivity in SN1 is 3º > 2º > 1º.
|
446 docs|929 tests
|