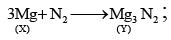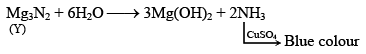JEE Advanced (Single Correct Type): The s-Block Element | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. The chemical formula of baking powder is ______
(a) NaHCO3
(b) Na2CO3
(c) Na2SO4
(d) NaCl
Correct Answer is option (a)
The chemical formula of baking powder is NaHCO3
Q.2. When the washing soda is heated
(a) CO is released
(b) CO + CO2 is released
(c) CO2 is released
(d) water vapours are released
Correct Answer is option (d)
upon heating, washing soda loses water molecules and becomes anhydrous sodium carbonate which does not decompose upon further heating.
Q.3. Which of the following metal ions plays an important role in muscle contraction?
(a) K+
(b) Na+
(c) Mg2+
(d) Ca2+
Correct Answer is option (d)
Ca2+ ions are involved in muscle contraction.
Q.4. Which of the following alkaline earth metal ions has the highest ionic mobility in aqua solution?
(a) Be2+
(b) Ca2+
(c) Ba2+
(d) Mg2+
Correct Answer is option (c)
The ionic mobility Is the maximum in Ba2+ ions since it has the least tendency to get hydrated.
Q.5. As the alkaline earth metal (except Be) tend to lose their valence electron readily they act as:
(a) weak oxidizing agents
(b) weak reducing agents
(c) strong oxidizing agents
(d) strong reducing agents
Correct Answer is option (d)
The alkaline earth metal (except Be) tends to lose its valence electrons readily and they act as strong reducing agents.
Q.6. Which has the maximum electropositive character?
(a) Cu
(b) Cs
(c) Ba
(d) Cr
Correct Answer is option (b)
Cs being an alkali metal is maximum electropositive in nature.
Q.7. Which sulfate has the highest solubility in water?
(a) BaSO4
(b) CaSO4
(c) BeSO4
(d) MgSO4
Correct Answer is option (c)
BeSO4 has the highest solubility due to maximum hydration energy.
Q.8. The correct order sequence of of the increasing covalent character is:
(a) LiCl < NaCl < BeCl2
(b) BeCl2 < LiCl < NaCl
(c) NaCl < LiCl < BeCl2
(d) BeCl2 < NaCl < LiCl
Correct Answer is option (c)
According to the Faizan rule greater the size of cation more is the ionic character, greater the charge on cation more is the covalent character. so the correct order of covalent character of the given compounds are NaCl < LiCl < BeCl2
Q.9. Which one of the alkali metals form only the normal oxide M2O on heating in air?
(a) Rb
(b) K
(c) Li
(d) Na
Correct Answer is option (c)
Lithium forms normal oxide Li2O.
Q.10. The alkaline earth metal present in chlorophyll is ______
(a) Be
(b) Mg
(c) Se
(d) Ba
Correct Answer is option (b)
The alkaline earth metal present in chlorophyll is Magnesium (Mg).
Q.11. A metal (X) on heating in nitrogen gas gives (Y). (Y) on treatment with H2O gives a colourless gas, which when passed through CuSO4 solution gives a blue colour. Identify the compound (Y).
(a) Mg(NO3)2
(b) Mg3N2
(c) NH3
(d) MgO
Correct Answer is option (b)
Q.12. The lattice energy order for lithium halides is
(a) LiF > LiCl > LiBr > LiI
(b) LiCl > LiF > LiBr > LiI
(c) LiBr > LiCl > LiF > LiI
(d) LiI > LiBr > LiCl > LiF
Correct Answer is option (a)
Smaller is the size of anion, lesser is its polarization, more is ionic nature, more is lattice energy.
Q.13. 1 mol of substance (X) was treated with an excess of water. 2 mol of readily combustible gas were produced alongwith solution which when reacted with CO2 gas produced a white turbidity. The substance (X) could be:
(a) Ca
(b) CaH2
(c) Ca(OH)2
(d) Ca(NO3)2
Correct Answer is option (b)
CaH2 + 2H2O → Ca(OH)2 + 2H2
Ca(OH)2 + CO2 → CaCO3 + H2O
Q.14. Based on lattice energy and other considerations which one of the following alkali metal chlorides is expected to have the highest melting point
(a) LiCl
(b) NaCl
(c) KCl
(d) RbCl
Correct Answer is option (b)
Although lattice energy of LiCl higher than NaCl but LiCl is covalent in nature and NaCl ionic thereafter, the melting point decreases as we move NaCl because the lattice energy decreases as a size of alkali metal atom increases (lattice energy ∝ melting point of alkali metal halide)
Q.15. The reagent commonly used to determine hardness of water titrimetrically is
(a) Oxalic acid
(b) Disodium salt of EDTA
(c) Sodium citrate
(d) Sodium thiosulphate
Correct Answer is option (b)
It form calcium and magnesium complex with EDTA
|
446 docs|929 tests
|