JEE Advanced (Subjective Type Questions): Hydrocarbons- 1 | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q. 1. Give one characteristic test which would distinguish. CH4 from C2H2 (1979)
Ans. Sol. Bromine water test : C2H2 decolourises bromine water while CH4 does not decolourises bromine water.
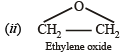
Q. 2. Write the structural formula of the major product in each of the following cases : (i) the compound obtained by the hydration of ethyne is treated with dilute alkali (1981 - ½ Mark) (ii) ethene mixed with air is passed under pressure over a silver catalyst at 250ºC. (1981 - ½ Mark)
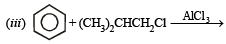
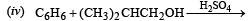
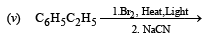
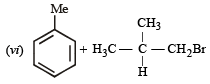
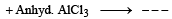
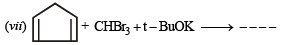
 (2000 - 1 Mark)
(2000 - 1 Mark)
Ans. Sol.
(i) 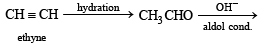
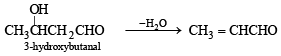
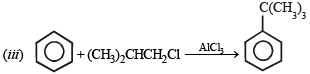
NOTE : that the 1° carbocation, 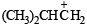 formed during reaction rearranges to the more stable, 3° carbocation,
formed during reaction rearranges to the more stable, 3° carbocation, 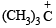 and hence the above product isformed.] (see also ix part)
and hence the above product isformed.] (see also ix part)
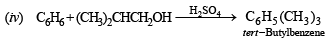
Explanation :
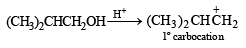
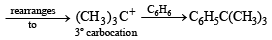
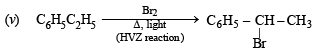
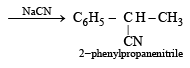
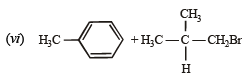
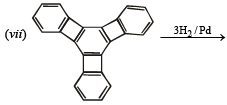
(vii) 
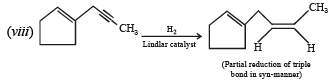
Q. 3. Outline the reaction sequence for the conversion of ethene to ethyne (the number of steps should not be more than two). (1981 - 1 Mark)
Ans. Sol. 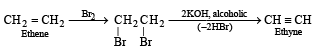
Q. 4. State with balanced equations, what happens when propene is bubbled through a hot aqueous solution of potassium permanganate. (1982 - 1 Mark)
Ans. Sol. 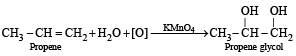
[NOTE : Colour of KMnO4 is discharged]
Q. 5. Give reasons for the following : (i) Methane does not react with chlorine in the dark. (1983 - 1 Mark)
(ii) Propene reacts with HBr to give isopropyl bromide but does not give n-propyl bromide. (1983 - 1 Mark)
(iii) Although benzene is highly unsaturated, normally it does not undergo addition reaction. (1983 - 1 Mark)
(iv) Toluene reacts with bromine in the presence of light to give benzyl bromide while in presence of FeBr3 it gives p-bromotoluene. Give explanation for the above obser vations. (1996 - 2 Marks)
(v) The central carbon-carbon bond in 1, 3 – butadiene is shorter than that in n-butane. (1998 - 2 Marks)
(vi) tert-Butylben zen e does n ot give ben zoic acid on treatment with acidic KMnO4. (2000 - 1 Mark)
 (2005 - 1 Mark)
(2005 - 1 Mark)
Ans. Sol. (i) TIPS/Formulae : Chlorination of methane is a free radical substitution reaction.
In dark, chlorine is unable to be converted into free radicals, hence the reaction does not occur.
(ii) TIPS/Formulae : Addition of unsymmetrical addendum (HBr in present case) to unsymmetrical olefin (CH3CH = CH2, in present case) takes place according to Markownikoff rule.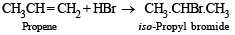
(iii) Unlike olefins, p-electrons of benzene are delocalised (resonance) and hence these are unreactive towards addition reactions. Moreover, addition reaction leads to destruction of the benzenoid ring. (iv) In presen ce of li gh t, toluene un dergoes side chain bromination through a free radical mechanism.
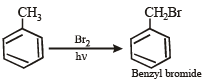
In presence of FeBr3, toluene undergoes electrophilic substitution in the benzene ring.
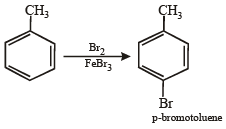
[NOTE :–CH3 is o-, p-directing]
(v) TIPS/Formulae : 1, 3 - Butadiene is a conjugated diene and is a reasonance hybrid:
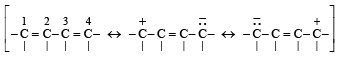
Thus resonance induces some double bond character in the central C-C bond leading to the shortening of this bond. Alternatively, all the four C atoms of 1, 3– butadiene are sp2 hybridised and thus their C – C bond length will be lower than that of n- butane in which all the four C atoms are sp3 hybridised.
(vi) tert-Butyl benz en e does n ot gi ve ben zoic a cid on treatment with acidic KMnO4 because it does not contain any hydrogen atom on the key carbon atom.
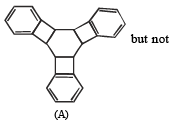
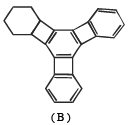
(vii) Reduction of cental ring to form A involves reduction of all the three cyclobutadiene rings (which are antiaromatic as they have 4p electrons each), i.e. antiaromatic rings are converted into nonaromatic rings.
On the other hand, reduction of the terminal ring to form B involves reduction of only one antiaromatic ring.
Remember that antiaromatic rings impart unstability.
Q. 6. (i) 2-Methylpropene can be converted into isobutyl bromide by hydrogen bromide, is true under what condition s? (1984 - 1 Mark)
(ii) 'Ethyne and its derivatives will give white precipitate with ammonical silver nitrate solution', is true under what conditions. (1984 - 1 Mark)
Ans. Sol. (i) NOTE : Under normal conditions, ter-butyl bromide is formed, isobutyl bromide is formed in presence of peroxide.
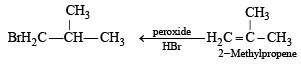
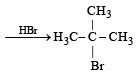
(ii) Ethyne (HC ≡ CH) and only those derivatives which have at least one acetylenic hydrogen atom (≡ C – H) i.e. terminal alkynes will give white precipitate with ammonical silver nitrate solution.
Q. 7. Write down the reactions involved in the preparation of the following, using the reagents indicated against it in parenthesis.
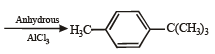
Ethylbenzene from benzene [C2H5OH, PCl5, anhydrous AlCl3]. (1984 - 2 Marks)
Ans. Sol.
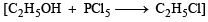
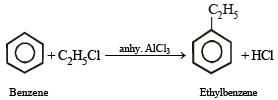
Q. 8. A certain hydrocarbon A was found to contain 85.7 percent carbon and 14.3 per cent hydrogen. This compound consumes 1 molar equivalent of hydrogen to give a saturated hydrocarbon B. 1.00 g of hydrocarbon A just decolourized 38.05 g of a 5 per cent solution (by weight) of Br2 in CCl4. Compound A, on oxidation with concentrated KMnO4, gave compound C (molecular formula C4H8O) and acetic acid. Compound C could easily be prepared by the action of acidic aqueous mercuric sulphate on 2- butyne. Determine the molecular formula of A and deduce the structure of A, B and C. (1984 - 6 Marks)
Ans. Sol. Calculation of molecular formula of A.

∴ Empirical formula of A = CH2 Determination of molecular weight of A
1g of A consumes = 38.05 g of 5% Br2 (in CCl4)
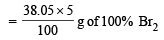
= 1.90 g of 100% Br2.
Now since 1.90 g of Br2 is consumed by 1 g of compound A
∴ 160g (1 mole) of Br2 will be consumed by
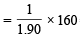 = 84.2 g of A = 84.0 (app.)g of A
= 84.2 g of A = 84.0 (app.)g of A
∴ Molecular weight of A = 84
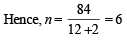
∴ Molecular formula of A = (CH2)6 = C6H12
Since the hydrocarbon A consumes 1 molar equivlaent of hydrogen, it must contain one double bond. Oxidation of compound A with KMnO4 to form compound C (C4H8O) and acetic acid indicates = CH.CH3 fragment in A, i.e.
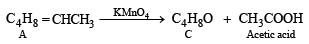
Now the fragment C4H8 of A on oxidation forms the compound 'C' (C4H8O) which may be easily obtained from butyne-2 and acidic aq. HgSO4, the compound 'C' must be ethylmethyl ketone.
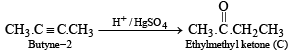
The formation of ketone 'C' from C4H8 fragment of 'A' can be explained by the following structure of A
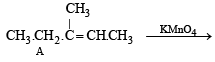
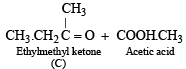
Hence formation of 'B' can be represented as below.
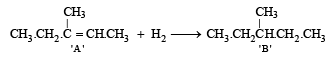
Q. 9. How would you distinguish between (i) 2-butyne and 1-butyne. (1985 - 1 Mark) (ii) cyclohexane and cyclohexene. (1988 - 1 Mark)
Ans. Sol. (i) By amm. AgNO3 or by acidic-H tests : Terminal alkynes give white precipitate with amm. AgNO3 or red ppt. with amm. Cu2Cl2 (H atom attached on sp hybridized carbon is acidic). 2CH3CH2C ≡ CH + Ag2O → 2CH3CH2C ≡ CAg + H2O
CH3 – C ≡ C – CH3 + Ag2O → No reaction
NOTE : Only terminal alkynes respond to these reactions. (ii) Cyclohexene gives positive response to bromine water test and Baeyer’s test while cyclohexane does not respond to these reagents.
Q. 10. n-Butane is produced by the monobromination of ethane followed by the Wurtz reaction. Calculate the volume of ethane at NTP required to produce 55 g n-butane, if the bromination takes place with 90 per cent yield and the Wurtz reaction with 85 per cent yield. (1989 - 3 Marks)
Ans. Sol. 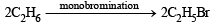 (yield 90%) (given)
(yield 90%) (given)
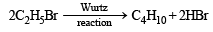 (yield 85%) (given)Moles of n-butane to be produced
(yield 85%) (given)Moles of n-butane to be produced
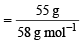 = 0.948 mol (∵ molecular mass of C4H10 = 58)
= 0.948 mol (∵ molecular mass of C4H10 = 58)
Amount of C2H5Br required to obtain 0.948 mol. of C4H10 = 2 × 0.948 mol.
Hence, the amount of C2H5Br required
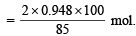 ...(1) [∵ yield is 85% only]
...(1) [∵ yield is 85% only]
Further 1 mole of C2H6 gives one mole of C2H5Br, hence number of moles of C2H6 reqd. for C2H5Br in (1)
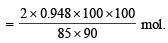 = 2.48 mol [∵ yield is 90%]
= 2.48 mol [∵ yield is 90%]
∴ Required volume of ethane at NTP = 22400 × 2.48 = 55552 ml. = 55.55 litres
Q. 11. Identify, B(C4H8) which adds on HBr in the presence and in the absence of peroxide to give the same product, C4H9Br. (1993 - 1 Mark)
Ans. Sol. TIPS/Formulae : A symmetric alkene does not follow Markovnikoff and antiMarkovnikoff 's rule (Peroxide effect).
B has to be a symmetric alkene (butene-2) CH3CH = CHCH3 as it will give the same product CH3 – CH(Br) – CH2– CH3 in presence /absence of peroxide.
Q. 12. Identify, D(C6H12), an optically active hydrocarbon which on catalytic hydrogenation gives an optically inactive compound, C6H14. (1993 - 1 Mark)
Ans. Sol. An optically active hydrocarbon will have an asymmetric C-atom. This means D(C6H12) should have an asymmetric C-atom & C6H14 will have no asymmetric C-atom, hence D would be 3-methylpentene-1,
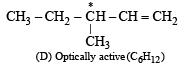
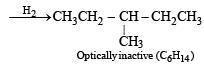
Q. 13. Draw the stereochemical structures of the products in the following reactions : (1994 - 4 Marks)
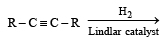
Ans. Sol. (i) SN2 reaction leads to inversion in configuration.
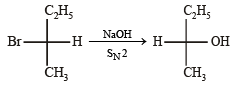
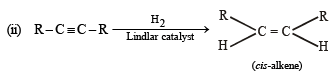
NOTE :
(i) Lindlar's catalyst is Pd supported over CaCO3 which is partially poisoned by (CH3COO)2Pb. It can restrict the hydrogenation of alkyne to alkene stage. It yields a cis-alkene. (ii) Reduction of alkynes to alkene stage can also be carried out with sodium or lithium in liquid NH3. Here transalkene is major product.
|
446 docs|929 tests
|
















