JEE Advanced Previous Year Questions (2019 - 2023): Chemical Bonding and Molecular Structure | Chemistry for JEE Main & Advanced PDF Download
2023
Q1: The correct molecular orbital diagram for F2 molecule in the ground state is : [JEE Advanced 2023 Paper 2]
(a)
(b)
(c)
(d)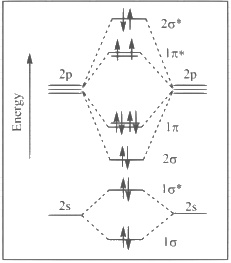
Ans: (c)
(1) Upto 14 electrons, molecular orbital configuration is -> Nb = No of electrons in bonding molecular orbital
Nb = No of electrons in bonding molecular orbital
Na = No of electrons in anti bonding molecular orbital
Here Na = Anti bonding electron = 4 and Nb = 10
(2) After 14 electrons to 20 electrons molecular orbital configuration is -> Here Na = 10
Here Na = 10
and Nb = 10
Now from question, in F atom 9 electrons present, so in F2, 9 × 2 = 18 electrons present.
Molecular orbital configuration of F2(18 electrons) is
Q2: Among [I3]+, [SiO4]4−, SO2 Cl2 , XeF2, SF4, ClF3, Ni (CO)4 , XeO2 F2, [PtCl4]2− , XeF4 , and SOCl2 , the total number of species having sp3 hybridised central atom is ________. [JEE Advanced 2023 Paper 2]
Ans: 5
Q3: Consider the following molecules: Br3O8 , F2O, H2S4O6, H2S5O6 , and C3O2. Count the number of atoms existing in their zero oxidation state in each molecule.
Their sum is _______. [JEE Advanced 2023 Paper 2]
Ans: 6 Number of atoms with zero oxidation state =0
Number of atoms with zero oxidation state =0 Number of atom with zero oxidation state = 0
Number of atom with zero oxidation state = 0 Number of atoms where zero oxidation state = 3
Number of atoms where zero oxidation state = 3 Number of atoms with zero oxidation state =1
Number of atoms with zero oxidation state =1
∴ Total atom with zero oxidation number state are 6.
2020
Q1: With respect to hypochlorite, chlorate and perchlorate ions, choose the correct statement(s). [JEE Advanced 2020 Paper 1]
(a) The hypochlorite ion is the strongest conjugate base.
(b) The molecular shape of only chlorate ion is influenced by the lone pair of electrons of Cl.
(c) The hypochlorite and chlorate ions disproportionate to give rise to identical set of ions.
(d) The hypochlorite ion oxidises the sulphite ion.
Ans: (a, b, d)
(a) Order of acid strength different oxyacids of chlorine are : Weak acid have strong conjugate base thus hypochlorite ion has strongest conjugate base. Therefore, statement (a) is correct.
Weak acid have strong conjugate base thus hypochlorite ion has strongest conjugate base. Therefore, statement (a) is correct.
(b) Hypochlorite ion is linear and perchlorate ion is tetrahedral and there is no effect of lone pair on hypochlorite ion. Thus, statement (b) is correct. (c) In the disproportionation reaction, chlorate ion Cl(+5) is oxidised to perchlorate, Cl(+7) and reduce to chloride, Cl(−1).
(c) In the disproportionation reaction, chlorate ion Cl(+5) is oxidised to perchlorate, Cl(+7) and reduce to chloride, Cl(−1).
While in hypochlorite ion, chlorite ion Cl(+1) is oxidised to chlorate, Cl(+5) and reduced to chloride, Cl(−1) ion.
Thus, statement (c) is incorrect.
(d) The hypochlorite ion oxidises the sulphite ion to sulphate ion, because HOCl is the strongest oxidising Cloxyacids,
Thus, statement (d) is correct.
Q2: Consider the following compounds in the liquid form :
O2, HF, H2O, NH3, H2O2, CCl4, CHCl3, C6H6, C6H5Cl
When a charged comb is brought near their flowing stream, how many of them show deflection as per the following figure? [JEE Advanced 2020 Paper 2] Ans: 6
Ans: 6
Only polar liquid will be attracted towards charged comb due to the formation of electrically charged droplets in the polar liquid stream, induced by a nearby charged object. Hence, liquid showing deflection are HF, H2O, NH3, H2O2, CHCl3, C6H5Cl.
Q3: The structure of a peptide is given below. If the absolute values of the net charge of the peptide at pH = 2, pH = 6, and pH = 11 are and |Z3|, respectively, then what is |Z1| + |Z2| + |Z3| ? [JEE Advanced 2020 Paper 2]
If the absolute values of the net charge of the peptide at pH = 2, pH = 6, and pH = 11 are and |Z3|, respectively, then what is |Z1| + |Z2| + |Z3| ? [JEE Advanced 2020 Paper 2]
Ans: 5
At pH = 2,
There are two − NH2 group, and + 1 charge on each group because all amino groups exist in the form of − NH3⊕.
Therefore, |Z1| = 2.
At pH = 6,
NH2 of lysine (+ 1) (pH = 9.47) and COOH (− 1) of glutamic (pH = 3.08) acid, so because of dipolar ion exists, therefore |Z2| = 0.
At pH = 11,
COOH of glutamic acid has (− 1), COOH of lysine (− 1) and OH of phenol (− 1).
Therefore, |Z3| = | − 3| = 3 (All COOH and OH exist in the form of − COO − and − O −).
∴ |Z1| + |Z2| + |Z3| = 2 + 0 + 3 = 5
2019
Q1: Each of the following options contains a set of four molecules. Identify the option(s) where all four molecules posses permanent dipole moment at room temperature. [JEE Advanced 2019 Paper 1]
(a) SO2, C6H5Cl, H2Se, BrF5
(b) BeCl2, CO2, BCl3, CHCl3
(c) NO2, NH3, POCl3, CH3Cl
(d) BF3, O3, SF6, XeF6
Ans: (a, c)
The molecules which gives permanent dipole moment are polar in nature.

|
352 videos|596 docs|309 tests
|
FAQs on JEE Advanced Previous Year Questions (2019 - 2023): Chemical Bonding and Molecular Structure - Chemistry for JEE Main & Advanced
| 1. What is the hybridization of the central atom in the molecule H2O? |  |
| 2. How many lone pairs are present on the central atom in the molecule NH3? |  |
| 3. What is the shape of the molecule CH4 based on VSEPR theory? |  |
| 4. How many sigma bonds are present in the molecule H2CO? |  |
| 5. What is the dipole moment of a molecule and how is it related to its symmetry? |  |
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|

















