Grade 9 Exam > Grade 9 Notes > AP Chemistry > Kinetic Particle Theory
Kinetic Particle Theory | AP Chemistry - Grade 9 PDF Download
| Table of contents |

|
| Solids |

|
| Liquids |

|
| Gases |

|
| Properties of Solids, Liquids and Gases |

|
Solids
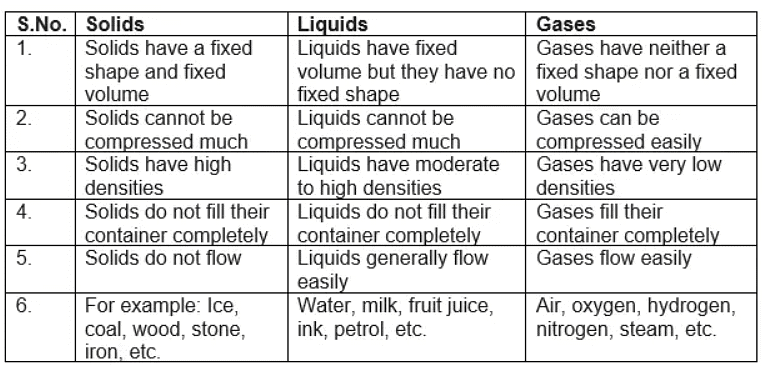
- Solids have a fixed volume and shape with a high density. The atoms vibrate in place but cannot change their positions. The particles are densely packed in a regular pattern.
- Solids maintain a fixed volume and shape with high density.
- Atoms in solids vibrate in position without moving.
- The particles in solids are tightly packed in a fixed and orderly manner.
Liquids
- Liquids have a fixed volume but take the shape of their container. They are generally less dense than solids (with water being an exception) but denser than gases.
- Liquids maintain a fixed volume but adapt to the shape of the container.
- They are usually less dense than solids (except water) but denser than gases.
- Particles in liquids move and slide past each other, allowing liquids to flow and take the shape of their container.
Question for Kinetic Particle TheoryTry yourself: Which of the following statements is true about solids?View Solution
Gases
- Gases do not have a fixed volume and, like liquids, take up the shape of the container.
- Gases have a very low density.
- Since there is a lot of space between the particles, gases can be compressed into a much smaller volume.
- The particles are far apart and move randomly and quickly (around 500 m/s) in all directions.
- They collide with each other and with the sides of the container (this is how pressure is created inside a can of gas).
Properties of Solids, Liquids and Gases
The document Kinetic Particle Theory | AP Chemistry - Grade 9 is a part of the Grade 9 Course AP Chemistry.
All you need of Grade 9 at this link: Grade 9
|
69 videos|92 docs|38 tests
|
FAQs on Kinetic Particle Theory - AP Chemistry - Grade 9
1. What is the Kinetic Particle Theory?$#
Ans. The Kinetic Particle Theory is a scientific theory that explains the behavior of particles in solids, liquids, and gases. It states that all particles are in constant motion, with different levels of energy depending on the state of matter they are in.
2. How do the properties of solids, liquids, and gases differ according to the Kinetic Particle Theory?$#
Ans. According to the Kinetic Particle Theory, solids have particles that are closely packed together and have little freedom of movement. Liquids have particles that are more spread out and can move past each other, while gases have particles that are far apart and have the most freedom of movement.
3. What are some examples of each state of matter based on the Kinetic Particle Theory?$#
Ans. Examples of solids include ice, wood, and metal. Examples of liquids include water, oil, and milk. Examples of gases include oxygen, carbon dioxide, and helium.
4. How does temperature affect the behavior of particles in solids, liquids, and gases according to the Kinetic Particle Theory?$#
Ans. As temperature increases, the particles in solids, liquids, and gases gain more energy and move faster. In solids, this can lead to melting into a liquid state. In liquids, this can lead to boiling into a gas state.
5. How does pressure impact the properties of gases according to the Kinetic Particle Theory?$#
Ans. Increasing the pressure on a gas causes the gas particles to be pushed closer together, resulting in a decrease in volume. This is because the particles have less space to move around in, leading to a higher density and more collisions between particles.
Ans. The Kinetic Particle Theory is a scientific theory that explains the behavior of particles in solids, liquids, and gases. It states that all particles are in constant motion, with different levels of energy depending on the state of matter they are in.
2. How do the properties of solids, liquids, and gases differ according to the Kinetic Particle Theory?$#
Ans. According to the Kinetic Particle Theory, solids have particles that are closely packed together and have little freedom of movement. Liquids have particles that are more spread out and can move past each other, while gases have particles that are far apart and have the most freedom of movement.
3. What are some examples of each state of matter based on the Kinetic Particle Theory?$#
Ans. Examples of solids include ice, wood, and metal. Examples of liquids include water, oil, and milk. Examples of gases include oxygen, carbon dioxide, and helium.
4. How does temperature affect the behavior of particles in solids, liquids, and gases according to the Kinetic Particle Theory?$#
Ans. As temperature increases, the particles in solids, liquids, and gases gain more energy and move faster. In solids, this can lead to melting into a liquid state. In liquids, this can lead to boiling into a gas state.
5. How does pressure impact the properties of gases according to the Kinetic Particle Theory?$#
Ans. Increasing the pressure on a gas causes the gas particles to be pushed closer together, resulting in a decrease in volume. This is because the particles have less space to move around in, leading to a higher density and more collisions between particles.
Related Searches















