Class 9 Exam > Class 9 Notes > Lab Manual: Solution, Colloids & Suspension
Lab Manual: Solution, Colloids & Suspension - Class 9 PDF Download
Objective
To prepare:
- a true solution of common salt, sugar and alum in water.
- a suspension of soil, chalk powder and fine sand in water.
- a colloidal solution of starch in water and egg albumin in water and distinguish between these on the basis of transparency, filtration criterion & stability
Theory
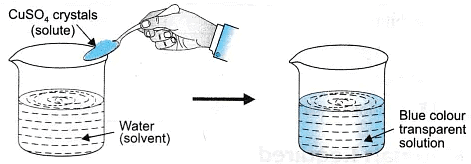
- True solution: A solution that has solute particles of size smaller than 1 nm (10-9 metres) in diameter, and cannot be seen with naked eyes. They do not scatter a beam of light, the particles do not separate by filtration and the particles do not settle down.
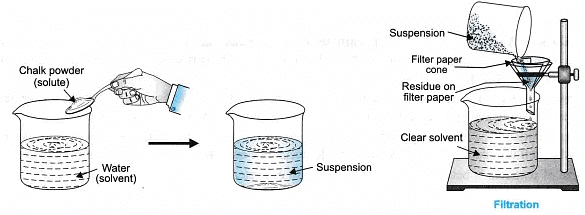
- Suspension: It is a heterogeneous mixture in which solute particles do not dissolve but remain suspended, particles can be seen with naked eyes, it scatters a beam of light, particles can be separated from the mixture by filtration.
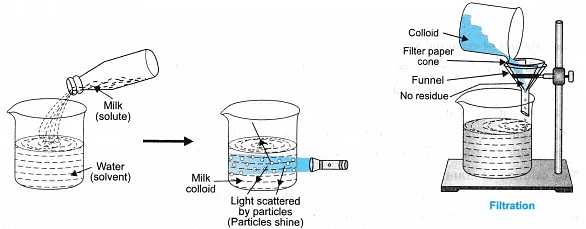
- Colloidal solution: The solution appears to be homogeneous, the particles can scatter a beam of light, they do not settle down when left undisturbed, it is stable and particles cannot be seen by naked eyes. The particles cannot be filtered. The size of particles is between 10-7 cm to 10-4 cm in diameter.
Properties of True Solutions
- A true solution is a homogeneous mixture of solute and solvent.
- The particle size of solute is less than 1 nm (1 nm =10-9m).
- The components do not scatter light and do not show Tyndall effect.
- The particles cannot be separated by filtration.
- The solution is stable (remains uniform).
- The solution is transparent.
Properties of Colloid
- It is a heterogeneous solution but appears to be homogeneous.
- The particle size of solute is 1 nm-1000 nm. (10-9-10-6 m)
- The components scatter light and shows Tyndall effect.
- The particles can be separated only by centrifugation.
- The solution is stable.
- The solution is translucent.
Properties of Suspension
- It is a heterogeneous mixture.
- Particle size is more than 1000 nm (10-6 m) and can be seen with naked eyes.
- The particles of suspension, in its suspended form scatter a beam of light, i.e., shows Tyndall effect.
- It is unstable.
- The particles can be separated by filtration.
- It is opaque.
Materials Required
Beakers (250 mL), an iron stand, a glass rod, bunsen burner, test tube stand, three funnels, three tripod stands, filter papers, a small torch and China dish.
Chemicals Required
Common salt, sugar crystals, alum powder, chalk powder, fine sand, raw egg, fine soil from garden and distilled water.
Procedure
1. To prepare a true solution of common salt, sugar and alum in water.- True solution of common salt:
Take a clean and dry beaker, pour 100 mL of distilled water in it and add dry common salt in it. Stir the content with a glass rod. Common salt dissolves completely to form a true solution. - True solution of sugar:
Take a clean and dry beaker and add 100 mL of distilled water in it, pour few sugar crystals in it arid stir the content with a glass rod. The sugar dissolves in water to form true solution. - True solution of alum:
Take a clean and dry beaker, add 100 mL of distilled water in it and add a pinch of alum powder, stir with a glass rod. The alum dissolves in water to form a true solution.
2. To prepare a suspension of soil, chalk powder and fine sand in water.
- Suspension of sand in water:
Take 100 mL of distilled water in a beaker, add 10 g of fine sand in it. Stir well using a glass rod. Allow it to stand for some time and record your observation.
(It does not dissolve in water) - Suspension of chalk powder in water:
Take 100 mL of distilled water in a beaker, add 10 g of chalk powder in it. Stir well and record your observation.
(chalk + water forms a suspension) - Suspension of soil in water:
Take 100 mL of distilled water in a beaker and 10 g of garden soil to it. Stir the mixture with a glass rod. Allow it to stand for sometime record your observation.
(Soil does not dissolve in water but forms a suspension)
3. To prepare colloidal solutions of starch and egg albumin in water.
- Colloidal solution of starch in water:
Take about 1 g of starch in a china dish, pour 10 mL of distilled water in the dish and stir the mixture. Now take 90 mL of hot, boiling water.
(Heat the water in beaker using Bunsen burner)
Stir the contents of the china dish continuously and pour the contents in the boiling water. Allow the contents to cool. Record your observation.
(The starch + water solution is colloidal in nature.) - Colloidal solution of egg albumin in water:
Take 10 ml of water in a beaker. Break an egg and discard the egg shells. Separate the white portion of egg from the yellow part. Add a very small quantity of egg albumin to it. Stir the contents thoroughly with the help of a glass rod. Then add 90 mL of distilled water with continuous stirring and few drops of dil. acid (dil. HCL, dil.H2S04). Record your observation.
(Egg albumin + water solution is colloidal in nature.)
4. To distinguish the above formed solutions on the basis of transparency, filtration criterion and stability:
- Take one true solution → salt + water
- Take one suspension → chalk + water
- Take one colloidal solution → egg albumin + water
- Transparency:
Take 3 test tubes with a colloid, suspension and true solution in each respectively. Paste a white paper with tick mark on one side of each test tube. Look at the tick mark through the contents of the three test tubes from the other side. Check for the transparency of tick mark. Record your observations. - Filtration Criterion:
Take 3 tripod stands, place a funnel over each stand. Fix the filter paper in the funnel and check for the filtration criterion. Record your observations.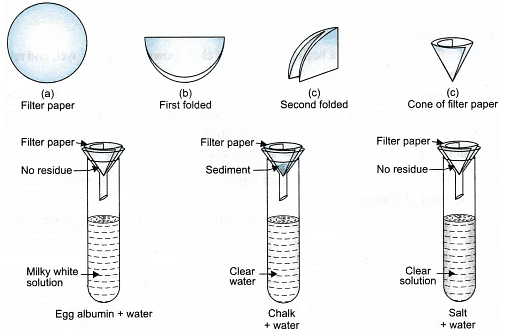
- Stability Criterion:
Take 3 test tubes with a colloid, suspension and true solution in each respectively. Shake all the test tubes and keep them in the test tube stand, allow it to stand for 5 minutes.
Record your observations.
- Transparency:
Observation Table
Precautions
- Use test tube holder for heating/boiling of water.
- Do not waste the chemicals and distilled water. Use it wisely.
- Always stir the contents in the test tube nicely and gently.
- Use only distilled water to make solutions.
Related Searches



















