Lakhmir Singh & Manjit Kaur: Chemical Effects of Electric Current- 2 | Lakhmir Singh & Manjit Kaur Solutions: Class 8 Science PDF Download
Short Answer Type Questions
Q.26. Which of the following liquids conduct electricity and which do not conduct electricity?
Lemon juice, Milk, Vinegar, Common salt solution, Sulphuric acid solution, Sugar solution, Distilled water, Honey, Sea water, Rainwater.
Lemon juice, Milk, Vinegar, common salt solution, Sulphuric acid solution, Sea water, and Rainwater conduct electricity.
Sugar solution, Distilled water and Honey do not conduct electricity.
Q.27. Why is it dangerous to touch a working electrical appliance with wet hands?
Because wet hands have water and water is a good conductor of electricity. A person can get electric shock if he touches any electrical appliances with wet hands.
Q.28. What is the advantage of using LED in testing the electrical conductivity of liquids?
The advantage of using LEDs is because, they glow up even if very small amount of current passes through them.
Q.29. Which effect of electric current is utilized for detecting the flow of current through a solution:
(a) when a torch bulb is used?
(b) when a compass is used?
(a) Heating effect of current is used detecting the flow of current through a solution.
(b) Magnetic effect of current is used detecting the flow of current through a solution.
Q.30. What happens to the needle of a compass kept nearby when electric current is switched on in a wire? Why does this happen?
The current carrying wire behaves like a magnet and it will cause deflection of the needle of the compass. This is called magnetic effect of electric current.
Q.31. Explain why, distilled water does not conduct electricity but tap water conducts some electricity.
Distilled water does not conduct electricity because it does not contain any ions which make it conducting. But it can conduct electricity when a pinch of common salt is added to it, as salt solution is conducting in nature.
Q.32. Distilled water does not conduct electricity. What substances can be added to distilled. water in small amounts to make it a good conductor of electricity? Why?
Lemon solution, salt, vinegar, sulphuric acid etc. can be added to added to distilled .water in small amounts to make it a good conductor of electricity. Because distilled water does not contain any ions which make it conducting. But adding these will provide free ions for conducting solution.
Q.33. Which of the two is a better conductor of electricity: drinking water (tap water) or sea water? Give reason for your answer.
Sea water is a better conductor because it contains sodium and chloride ions in large amounts. These ions make the solution conducting and thus, sea water is considered better conductor than the tap water which does not contain ions in large amounts.
Q.34. Why does a brand new bicycle have shining handlebar and wheel rims? What will happen if these are accidentaly scratched?
The shining coating is done on the metal by electroplating. When these are accidentaly scratched then, the metal will be rusted due to presence of air. So, by some metal these are coated so as to avoid rusting of iron mainly used to build the handlebars and wheel rims.
Q.35. Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
No, it is very risky and unsafe for the electrician to carry out electrical repairs outdoors during heavy downpour because rain water is conducting in nature as it contains dissolved salts. The electrician may get electrical shocks while working outdoors during rain as water may conduct electricity.
Q.36. When the free ends of a conductivity tester (made by using a battery connected to a wire wound around a compass) are dipped into a solution, the magnetic needle shows deflection. Can you give the reason for this deflection.
Because the free ends of a conductivity tester are dipped into a conducting solution then, it behaves like a current carrying wire and a current carrying wire behaves as a magnet because it produces magnetic field around it. And, due to this there is deflection in the compass.
Q.37. A beaker contains an acidified copper sulphate solution. A copper plate and a carbon rod are kept in this copper sulphate solution. The copper plate is connected to the positive terminal of a battery whereas the carbon rod is connected to the negative terminal of the battery. What will you observe when an electric current is passed through this set-up for a considerable time?
The carbon is a non-metal which is more reactive than copper. When, an electric current is passed through this set-up for a considerable time, then carbon replaces copper ions from solutions and they deposit on the negative electrode. Thus, a red-brown layer of copper metal will be deposited on the carbon rod connected to the negative terminal of the battery.
Q.38. Does pure water conduct electricity? If not, what can we do to make it conducting?
No. Pure water does not conduct electricity. Pure water does not contain any ions which make it conducting. But it can conduct electricity when a pinch of common salt is added to it, as salt solution is conducting in nature.
Q.39. In case of a fire, before the firemen use the water hoses to throw water to douse fire, they shut off the electricity supply for the area. Explain why this is done.
In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area because water may conduct electricity. If the electrical supply for the area is not shut off and water is poured over electrical appliances, then electricity may pass through water and harm the firemen.
Q.40.
(A) Which effect of electric current is utilized when a thin layer of chromium metal is deposited on an iron tap? What is this process known as?
This is called chemical effect of heating and the process is called as electroplating.
(B) For electroplating copper on an iron object, which terminal of the battery (positive or negative) is connected to the iron object? Also name the electrolyte you will use for this purpose.
In the process of electroplating, the metal to be electroplated is taken as cathode and the one used for coating is chosen as the anode. And, we know, anode is positive terminal and the cathode is negative terminal. Thus, negative terminal will be connected to the iron object.
Long Answer Type Questions
Q.41.
(A) What is meant by the chemical effect of electric current? Explain with the help of an example.
When electric current passes through any conducting solution, then chemical reactions take place inside the conducting solution. This effect is called as chemical effect of electric current. Example, electroplating. The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
(B) Name any two applications of the chemical effect of electric current.
- Chromium plating is done on metals body parts of cars, bikes, cycles, etc.
- Gold and silver plating is done on the artificial jewelry.
(C) What is electrolysis? Explain why, in the electrolysis of water, ‘acidified water’ is used.
Electrolysis is the process of dissociation of water molecules into H+ and OH– ions. When electric current Is passed through it in a conducting state. Because water does not have much higher concentration of ions. Dilute sulphuric acid is added to increase the concentration of ions in the water.
Q.42.
(A) Name three types of substances in which an electric current can produce a chemical effect.
The three types of substances in which an electric current can produce a chemical effect.
- Water
- Copper sulphate
- Vinegar.
(B) State some of the characteristics of chemical changes brought about by the chemical effect of electric current.
The some of the characteristics of chemical changes brought about by the chemical effect of electric current:
- The solution colour changes might take place.
- The bubbles evolve through the solution.
- The chemical deposition of one metal and replacement of the other metal.
(C) Why does an electric bulb glow when a current passes through it?
The electric bulb glows because it completes the circuit when put in the conducting solution and the conducting solution helps to glow the bulb.
Q.43.
(A) What is meant by electroplating? What is the purpose of electroplating?
The process of coating of a layer of any metal over other metal is called electroplating. The purpose of electroplating is to provide the shiny appearance, rust free metal surface etc.
Example:
- Chromium plating is done on metals body parts of cars, bikes, cycles, etc.
- Gold and silver plating is done on the artificial jewelry.
(B) Which properties of chromium metal make it suitable for electroplating it on car bumpers, bath taps and bicycle handlebars, etc., made of iron?
The chromium metal is suitable for electroplating it on car bumpers, bath taps and bicycle handlebars, etc., made of iron because it is corrosion resistant and also gives shiny appearance.
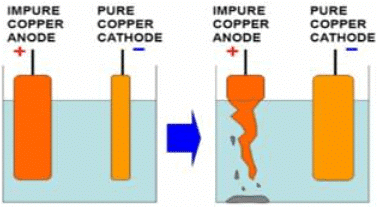
Q.44. A strip of impure copper metal is given to you. Describe briefly how you will purify it by using the chemical effect of electric current. Draw a labelled diagram of the experimental set up used for this purpose.
Copper ion is positively charged. It will be attracted towards the plate which is connected to the negative terminal of the battery. Because positive charges attract negative charges and repel positive charges.
As copper ions from the impure copper electrode are transferred to the thin copper plate, this thin pure copper plate must be connected to the negative terminal of the battery. Consequently, impure copper rod is connected to the positive terminal of the battery. In this way, pure copper is deposited on the negative electrode i.e. cathode.
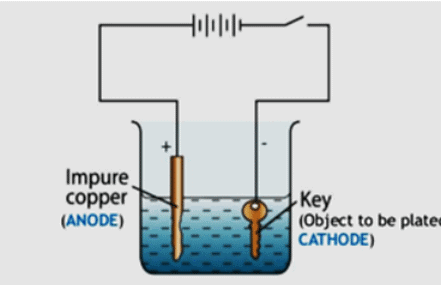
Q.45. With the help of a labelled diagram, describe briefly how an iron key can be electroplated with copper.
We need two electrodes made from different conducting materials (here copper and iron as anode and cathode respectively), an electrolyte, and electricity supply. The iron key will be taken as the cathode (negative electrode ) and the copper to be electroplated on the iron key to be made the anode (positive electrode). When we open the electricity, then copper ions are deposited on the negative electrode and in this way copper is electroplated on the iron key.