Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Limiting Reactants
Limiting Reactants | Chemistry for Grade 10 PDF Download
Limiting & Excess Reactant
Higher Tier Only- A chemical reaction does not go on indefinitely and stops when one of the reagents is used up
- The reagent that is used up first is the limiting reactant, as it limits the duration of the reaction and hence the amount of product that a reaction can produce
- The one that is remaining is the excess reactant
- The limiting reagent is the reactant which is not present in excess in a reaction
- The amount of product obtainable is therefore directly proportional to the amount of the limiting reagent added at the beginning of a reaction
- So, if you use half of the limiting reagent then you will get half of the product, provided the other reagents are present in excess. If you double the amount of the limiting reagent then you obtain double the amount of product
Determining the Limiting Reactant
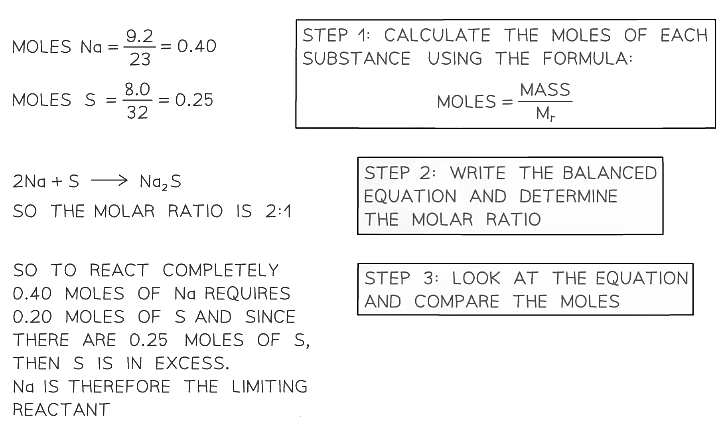
- In order to determine which reactant is the limiting reagent in a reaction, we have to consider the amounts of each reactant used and the molar ratio of the balanced chemical equation
- When performing reacting mass calculations, the limiting reagent is always the number that should be used, as it indicates the maximum possible amount of product that can form
- Once all of a limiting reagent has been used up, the reaction cannot continue
- The steps are:
- Convert the mass of each reactant into moles by dividing by the molar masses
- Write the balanced equation and determine the molar ratio
- Look at the equation and compare the moles
Worked Example
In a reaction to produce sodium sulfide, Na2S, 9.2 g of sodium is reacted with 8.0 g of sulfur. Which reactant is in excess and which is limiting?Exam Tip
- For a reactant to be present in excess, there only needs to be slightly more of it present than the other reactant, as determined from the molar ratio. In a two-reactant system, if one reactant is in excess then the other is by default the limiting reagent.
- A common error is to determine the limiting reactant as the reactant with the least amount of moles in the molar ratio. This is incorrect as the masses of each reactant must also be considered.
The document Limiting Reactants | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
73 videos|87 docs|21 tests
|
Related Searches