Chemistry Exam > Chemistry Notes > Physical Chemistry > Lindemann Mechanism & Types of Catalysis
Lindemann Mechanism & Types of Catalysis | Physical Chemistry PDF Download
- In chemical kinetics, the Lindemann mechanism (also called the Lindemann–Christiansen mechanism or the Lindemann–Hinshelwood mechanism) is a schematic reaction mechanism for unimolecular reactions. Frederick Lindemann and J. A. Christiansen proposed the concept almost simultaneously in 1921, and Cyril Hinshelwood developed it to take into account the energy distributed among vibrational degrees of freedom for some reaction steps.
- It breaks down an apparently unimolecular reaction into two elementary steps, with a rate constant for each elementary step. The rate law and rate equation for the entire reaction can be derived from the rate equations and rate constants for the two steps.
- The Lindemann mechanism is used to model gas phase decomposition or isomerization reactions. Although the net formula for a decomposition or isomerization appears to be unimolecular and suggests first-order kinetics in the reactant, the Lindemann mechanism shows that the unimolecular reaction step is preceded by a bimolecular activation step so that the kinetics may actually be second-order in certain cases.
Activated reaction intermediates
The overall equation for a unimolecular reaction may be written A → P, where A is the initial reactant molecule and P is one or more products (one for isomerization, more for decomposition).
- A Lindemann mechanism typically includes an activated reaction intermediate, labeled A*.
- The activated intermediate is produced from the reactant only after sufficient activation energy is acquired by collision with a second molecule M, which may or may not be similar to A. It then either deactivates from A* back to A by another collision, or reacts in a unimolecular step to produce the product(s) P.
The two-step mechanism is then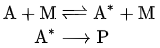
Rate equation in steady-state approximation
- The rate equation for the rate of formation of product P may be obtained by using the steady-state approximation, in which the concentration of intermediate A* is assumed constant because its rates of production and consumption are (almost) equal. This assumption simplifies the calculation of the rate equation.
- For the schematic mechanism of two elementary steps above, rate constants are defined as k1 for the forward reaction rate of the first step, k-1 for the reverse reaction rate of the first step, and k2 for the forward reaction rate of the second step.
- For each elementary step, the order of reaction is equal to the molecularity.
The rate of production of the intermediate A* in the first elementary step is simply: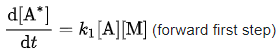
A* is consumed both in the reverse first step and in the forward second step. The respective rates of consumption of A* are: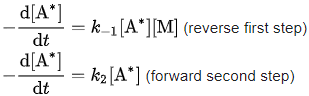
According to the steady-state approximation, the rate of production of A* equals the rate of consumption. Therefore: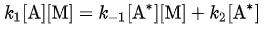
Solving for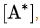 it is found that
it is found that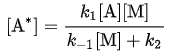
The overall reaction rate is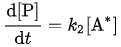
Now, by substituting the calculated value for [A*], the overall reaction rate can be expressed in terms of the original reactants A and M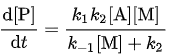
Reaction order and rate-determining step
- The steady-state rate equation is of mixed order and predicts that a unimolecular reaction can be of either first or second order, depending on which of the two terms in the denominator is larger.
- At sufficiently low pressures, k-1[M]≪ k2 so that d[P]/dt=k1[A][M], which is second order. That is, the rate-determining step is the first, bimolecular activation step.
At higher pressures, however, k-1[M]≫ k2 so that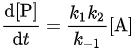 which is first order, and the rate-determining step is the second step, i.e. the unimolecular reaction of the activated molecule.
which is first order, and the rate-determining step is the second step, i.e. the unimolecular reaction of the activated molecule. - The theory can be tested by defining an effective rate constant (or coefficient) kuni which would be constant if the reaction were first order at all pressures:
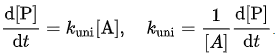
The Lindemann mechanism predicts that k decreases with pressure and that its reciprocal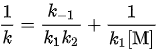 is a linear function of
is a linear function of 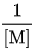 or equivalently of 1/p. Experimentally for many reactions, k does decrease at low pressure, but the graph of 1/k as a function of 1/p is quite curved.
or equivalently of 1/p. Experimentally for many reactions, k does decrease at low pressure, but the graph of 1/k as a function of 1/p is quite curved. - To account accurately for the pressure-dependence of rate constants for unimolecular reactions, more elaborate theories are required such as the RRKM theory.
Decomposition of dinitrogen pentoxide
- In the Lindemann mechanism for a true unimolecular reaction, the activation step is followed by a single step corresponding to the formation of products. Whether this is actually true for any given reaction must be established from the evidence.
- Much early experimental investigation of the Lindemann mechanism involved study of the gas-phase decomposition of dinitrogen pentoxide 2N2O5 → 2N2O4 + O2. This reaction was studied by Farrington Daniels and coworkers, and initially assumed to be a true unimolecular reaction.
- However, it is now known to be a multistep reaction whose mechanism was established by Ogg as:
N2O5 ⇌ NO2 + NO3
NO2 + NO3 → NO2 + O2 + NO
NO + N2O5 → 3NO2
- An analysis using the steady-state approximation shows that this mechanism can also explain the observed first-order kinetics and the fall-off of the rate constant at very low pressures.
Activity of Catalyst
- Catalyst has an ability to increase the rate of reaction. This ability of catalyst is known as the activity of catalyst.
- It depends upon adsorption of reactants on the surface of catalyst. Chemisorption is the main factor governing the activity of catalysts. The bond formed during adsorption between the catalytic surface and the reactants must not be too strong or too weak.
- It must be strong enough to make the catalyst active whereas, not so strong that the reactant molecules get immobilized on the catalytic surface leaving no further space for the new reactants to get adsorbed.
- Generally for the hydrogenation reaction, from Group 5 to Group 11 metals, the catalytic activity increases. The catalytic activity is found to be highest for group 7-9 elements of the periodic table.
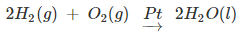
Selectivity of Catalyst
- Catalysts are highly specific compounds. They have an ability to direct the reaction to yield a particular product. The reaction with same reactants but different catalyst may yield different products. This is termed as the selectivity of catalyst. Catalysts are highly selective in nature.
- They can accelerate a particular reaction while inhibit another reaction. Hence, we can say a particular catalyst can catalyse one particular reaction only. It may fail to catalyse another reaction of the same type.
- For example: reaction of hydrogen and carbon monoxide yields methane when nickel is used as catalyst, methanol when a mixture of zinc oxide and chromium oxide is used as catalyst and methanal when only copper is used as catalyst.
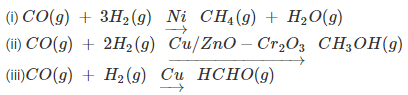
Types of catalysts
A catalyst is a chemical compound which makes the reaction occur more quickly by reducing the reaction’s activation energy barrier. During the reaction it isn’t eaten.
- Homogeneous catalyst – Homogeneous catalysts are usually soluble metal salts or compounds that are dissolved in an effective organic solvent that is used as the medium for reaction. (here catalyst and the reactants are on the same phase)
- Heterogeneous catalyst – A heterogeneous catalyst is a functional material which under conditions of reaction continually creates active sites with its reactants. (here catalyst and reactants are on different phase).
The document Lindemann Mechanism & Types of Catalysis | Physical Chemistry is a part of the Chemistry Course Physical Chemistry.
All you need of Chemistry at this link: Chemistry
|
84 videos|142 docs|67 tests
|
FAQs on Lindemann Mechanism & Types of Catalysis - Physical Chemistry
| 1. What is the Lindemann Mechanism? |  |
Ans. The Lindemann Mechanism is a chemical reaction mechanism that describes the steps involved in a catalytic reaction. It specifically explains the process of unimolecular reactions, where a single reactant molecule undergoes a chemical transformation. According to the Lindemann mechanism, the reaction occurs in two steps: first, the reactant molecule collides with the catalyst surface and forms an activated complex, and second, the activated complex decomposes to form the desired product.
| 2. What are the types of catalysis? |  |
Ans. There are three main types of catalysis:
1. Homogeneous Catalysis: In this type, the catalyst and the reactants are in the same phase, usually a liquid or a gas. The catalyst forms an intermediate complex with the reactants, facilitating the reaction.
2. Heterogeneous Catalysis: Here, the catalyst and the reactants are in different phases. The reactants are adsorbed onto the surface of the catalyst, where the reaction takes place. This type of catalysis is commonly used in industrial processes.
3. Enzyme Catalysis: Enzymes are biological catalysts that speed up chemical reactions in living organisms. They are highly specific and can catalyze a wide range of reactions. Enzyme catalysis occurs in aqueous environments and is crucial for various biological processes.
| 3. How does the Lindemann Mechanism explain catalysis? |  |
Ans. The Lindemann Mechanism provides a detailed explanation of catalysis in unimolecular reactions. It suggests that the reaction occurs in two steps: adsorption and decomposition. In the adsorption step, the reactant molecule collides with the catalyst surface and forms an activated complex. This complex is an intermediate state with higher energy and different geometry than the reactant molecule. In the decomposition step, the activated complex breaks down to form the desired product. The Lindemann Mechanism helps understand the role of catalysts in accelerating chemical reactions by providing a step-by-step explanation.
| 4. What are the advantages of heterogeneous catalysis? |  |
Ans. Heterogeneous catalysis offers several advantages:
1. Selectivity: Heterogeneous catalysts can be designed to selectively catalyze specific reactions, allowing the desired products to be obtained with high efficiency. This selectivity is crucial in industrial processes where the production of specific compounds is desired.
2. Reusability: Unlike homogeneous catalysts, heterogeneous catalysts can be easily separated from the reaction mixture, making them reusable. This reduces the cost and environmental impact of the catalytic process.
3. Stability: Heterogeneous catalysts are often more stable than their homogeneous counterparts, allowing them to withstand harsh reaction conditions and prolong their lifespan.
4. Ease of handling: Heterogeneous catalysts are typically in solid form, making them easier to handle and transport compared to liquid or gaseous catalysts.
| 5. How are enzymes different from other catalysts in terms of catalysis? |  |
Ans. Enzymes differ from other catalysts in several ways:
1. Specificity: Enzymes exhibit high specificity for their substrate molecules. Each enzyme is designed to catalyze a particular reaction or a group of closely related reactions, ensuring precision in biochemical processes.
2. Efficiency: Enzymes are incredibly efficient catalysts, often achieving rate enhancements of several orders of magnitude. They can accelerate reactions millions of times faster than the corresponding uncatalyzed reactions.
3. Regulation: Enzyme activity can be regulated through various mechanisms, allowing organisms to control the rate of specific reactions in response to environmental changes or metabolic needs.
4. Biocompatibility: Enzymes function under mild conditions, typically at physiological temperatures and pH levels. This biocompatibility is essential for their role in biological systems.
5. Enantioselectivity: Many enzymes exhibit enantioselectivity, meaning they can selectively catalyze reactions to produce a specific enantiomer of a chiral compound. This property is crucial in the synthesis of pharmaceuticals and other complex organic molecules.
Related Searches
















