Many Proteins are Enzymes | Biology for Grade 12 PDF Download
Enzymes as Proteins
- Enzymes are biological catalysts
- ‘Biological’ because they function in living systems
- ‘Catalysts’ because they speed up the rate of chemical reactions without being used up or changed
- Enzymes are also globular proteins
- Critical to the enzyme's function is the active site where the substrate binds
- Metabolic pathways are controlled by enzymes in a biochemical cascade of reactions
- Virtually every metabolic reaction within living organisms is catalysed by an enzyme – enzymes are therefore essential for life to exist
- Enzymes can be intracellular or extracellular referring to whether they are active inside or outside the cell respectively
- Intracellular enzymes are produced and function inside the cell
- Extracellular enzymes are secreted by cells and catalyse reactions outside cells (eg. digestive enzymes in the gut)

Mode of Enzyme Action
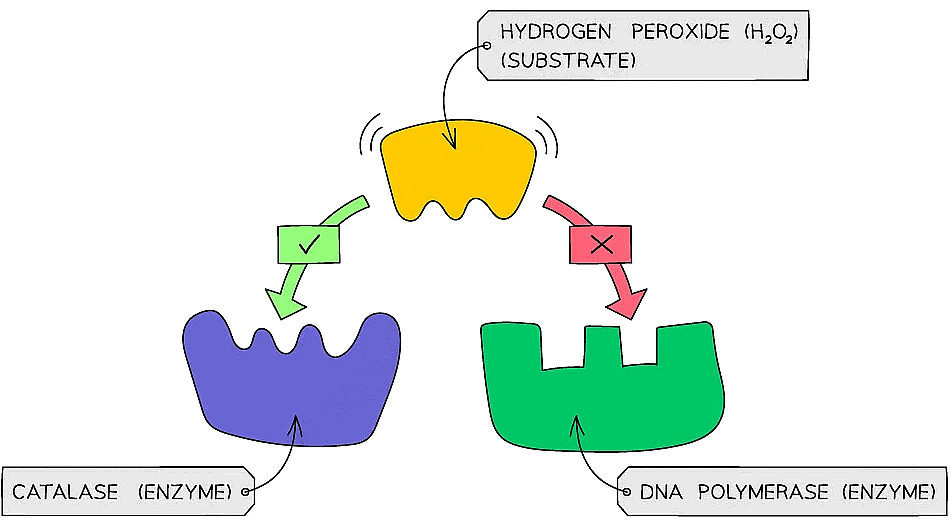
- Enzymes have an active site where specific substrates bind forming an enzyme-substrate complex
- The active site of an enzyme has a specific shape to fit a specific substrate
- Extremes of heat or pH can change the shape of the active site, preventing substrate binding – this is called denaturation
- Substrates collide with the enzymes active site and this must happen at the correct orientation and speed in order for a reaction to occur
 The active site of an enzyme has a specific shape to fit a specific substrate (when the substrate binds an enzyme-substrate complex is formed)
The active site of an enzyme has a specific shape to fit a specific substrate (when the substrate binds an enzyme-substrate complex is formed)
- The specificity of an enzyme is a result of the complementary nature between the shape of the active site on the enzyme and its substrate(s)
- The shape of the active site (and therefore the specificity of the enzyme) is determined by the complex tertiary structure of the protein that makes up the enzyme:
- Proteins are formed from chains of amino acids held together by peptide bonds
- The order of amino acids determines the shape of an enzyme
- If the order is altered, the resulting three-dimensional shape changes
- An enzyme-substrate complex forms when an enzyme and its substrate join together
- The enzyme-substrate complex is only formed temporarily, before the enzyme catalyses the reaction and the product(s) are released

- Enzyme reactions can either be catabolic or anabolic
- Catabolic reactions involve the breakdown of complex molecules into simpler products, which happens when a single substrate is drawn into the active site and broken apart into two or more distinct molecules
- Examples of catabolic reactions include cellular respiration and hydrolysis reactions

- Anabolic reactions involve the building of more complex molecules from simpler ones by drawing two or more substrates into the active site, forming bonds between them and releasing a single product
- Examples of anabolic reactions include protein synthesis and photosynthesis

Enzymes work by lowering the activation energy of a reaction
- All chemical reactions are associated with energy changes
- For a reaction to proceed there must be enough activation energy
- Activation energy is the amount of energy needed by the substrate to become just unstable enough for a reaction to occur and for products to be formed
- Enzymes speed up chemical reactions because they influence the stability of bonds in the reactants
- The destabilisation of bonds in the substrate makes it more reactive
- Enzymes work by lowering the activation energy of a reaction and in doing so they provide an alternative energy pathway

How Enzymes Work
The lock-and-key hypothesis
- Enzymes are globular proteins
- This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex tertiary structure of the protein that makes up the enzyme and is therefore highly specific
- In the 1890’s the first model of enzyme activity was described by Emil Fischer:
- He suggested that both enzymes and substrates were rigid structures that locked into each other very precisely, much like a key going into a lock
- This is known as the ‘lock-and-key hypothesis’
- This was later modified and adapted to our current understanding of enzyme activity, permitted by advances in techniques in the molecular sciences

The induced-fit hypothesis
- The modified model of enzyme activity is known as the ‘induced-fit hypothesis’
- Although it is very similar to the lock and key hypothesis, in this model the enzyme and substrate interact with each other:
- The enzyme and its active site (and sometimes the substrate) can change shape slightly as the substrate molecule enters the enzyme
- These changes in shape are known as conformational changes
- This ensures an ideal binding arrangement between the enzyme and substrate is achieved
- This maximises the ability of the enzyme to catalyse the reaction

Required Practical: Measuring Enzyme Activity
- The progress of enzyme-catalysed reactions can be investigated by:
- Measuring the rate of formation of a product using catalase
- Measuring the rate of disappearance of a substrate using amylase
Investigating catalase activity
- In this investigation, the rate of product formation is used to measure the rate of an enzyme-controlled reaction:
- Hydrogen peroxide is a common but toxic by-product of metabolism
- This means it must be broken down quickly
- Catalase is an enzyme found in the cells of most organisms that breaks down hydrogen peroxide into water and oxygen
- Hydrogen peroxide and catalase are combined and the volume of oxygen generated is measured in a set time
- The rate of reaction can then be calculated

Investigating amylase activity using iodine
- In this investigation, the rate of substrate disappearance is used to compare rates of reaction under different conditions
- Amylase is a digestive enzyme that hydrolyses starch into maltose and glucose
- Amylase functions best at pH 7 and 37oC (all enzymes operate best under specific conditions)
- Amylase and starch are combined and this reaction mixture is then tested for starch at regular time intervals
- This can be done by taking samples from the reaction mixture at each time interval and adding each sample to some iodine in potassium iodide solution
- Starch forms a blue-black colour with this solution
- If no starch is present, the iodine solution remains yellow-brown
- In this way, the time taken for starch to be broken down can be measured
- The investigation can be repeated under a variety of conditions (eg. by altering pH, temperature, enzyme concentration or starch concentration) and the reaction rates can then be compared

Investigating the effect of starch concentration on amylase activity using colourimetry
- A colourimeter is able to measure light absorbance (how much light is absorbed) or light transmission (how much light passes through) a substance
- Colourimetry can be used in any enzyme-catalysed reaction that involves colour change
- As the colour breaks down the transmission increases or light absorption decreases and this can be used to measure the rate of the reaction
- For example, a colourimeter can be used to follow the progress of a starch-amylase catalysed reaction as the amylase breaks the starch down into maltose
- This can be carried out as follows:
- Colourimeter calibration: this is an important step in a colourimetric investigation and in this case a weak iodine solution can be used to calibrate the colourimeter as the end point (or 100% transmission)
- Preparation of a starch solution of known concentration (stock solution), from which a range of concentrations are made using serial dilutions (method outlined in diagram below)
- Following calibration and switching on the red filter (to maximise the percentage transmission or absorbance), the colourimeter is used to measure the percentage absorbance or percentage transmission values
- Sometimes a reagent or indicator is used to produce the colours detected by the colourimeter and sometimes the solutions themselves absorb light waves
- A calibration graph is then plotted of starch concentration (X-axis) vs percentage absorbance or percentage transmission (Y-axis)

Drawing a Graph for Enzyme Rate Experiments
- Enzyme rate experiments are experiments that are carried out to determine the effect of changing a particular factor on the rate of a reaction that is catalysed by an enzyme
- Factors that can be changed include:
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
- The ways in which the reaction rate can be measured include:
- Measuring how much of a product is made in a given time period (e.g. using a gas cylinder to collect the oxygen produced from the breakdown of hydrogen peroxide by catalase)
- Measuring how much a substrate is broken down in a given time period (e.g. using iodine to determine how quickly starch is broken down into maltose by amylase)
- Line graphs should be used to present the results of enzyme rate experiments
- The data should be plotted with the independent variable on the x-axis and the dependent variable on the y-axis
- If a trend can be identified, a line of best fit (straight or curved) should be added to the graph
- If asked in an exam, you can use this line of best fit to interpolate (reading off values in between existing data points) or extrapolate (going beyond the range of existing data points to read off values)
- Using the graph and best fit line below, for example, you can use interpolation to conclude that, at a substrate concentration of 3 arbitrary units, the rate must have been 1.8 arbitrary units and you can use extrapolation to conclude that, at a substrate concentration of 10 arbitrary units, the rate will be 6 arbitrary units

- For some enzyme rate experiment graphs, it may be necessary to plot more than one set of data on the same graph
- For example, if investigating the effect of temperature on the rate of an enzyme-controlled reaction, you may need to plot a line graph with multiple lines, where each line represents the data collected at a specific temperature
- When drawing a graph like this, make sure to clearly label each line
- An example is shown below:
 The results of an enzyme experiment. It is the volume of product produced and not the rate of reaction that has been plotted on the y axis for this graph. The initial rate of reaction is represented by the initial gradient of the lines on the graph. As the temperature is the factor being manipulated it is the independent variable.
The results of an enzyme experiment. It is the volume of product produced and not the rate of reaction that has been plotted on the y axis for this graph. The initial rate of reaction is represented by the initial gradient of the lines on the graph. As the temperature is the factor being manipulated it is the independent variable.
Tips for plotting line graphs
- When plotting line graphs for enzyme rate experiments, remember the following:
- Plot data points accurately
- Use appropriate linear scales on axes
- Choose scales that enable all data points to be plotted within the graph area
- Label axes, with units included
- Make graphs that fill the space the exam paper gives you
- Draw a line (or curve) of best-fit to identify trends. The line must be smooth and have a balance of data points above and below the line
- In some cases, the line of best fit should be drawn through the origin, for example for rate–concentration graphs (the reaction cannot occur if the concentration of enzyme or substrate is 0). The line of best fit should only go through the origin if the data and trend allow it
Using a Tangent to Find Initial Rate of Reaction
- For linear graphs (i.e. graphs with a straight-line), the gradient is the same throughout
- This makes it easy to calculate the rate of change (rate of change = change ÷ time)
- However, many enzyme rate experiments produce non-linear graphs (i.e. graphs with a curved line), meaning they have an ever-changing gradient
- They are shaped this way because the reaction rate is changing over time
- In these cases, a tangent can be used to find the reaction rate at any one point on the graph:
- A tangent is a straight line that is drawn so it just touches the curve at a single point
- The slope of this tangent matches the slope of the curve at just that point
- You then simply find the gradient of the straight line (tangent) you have drawn
- The initial rate of reaction is the rate of reaction at the start of the reaction (i.e. where time = 0)
Example: The graph below shows the results of an enzyme rate reaction. Using this graph, calculate the initial rate of reaction.

Step 1: Estimate the extrapolated curve of the graph
Step 2: Find the tangent to the curve at 0 seconds (the start of the reaction)
The tangent drawn in the graph above shows that 72 cm3 of product was produced in the first 20 seconds.
Step 3: Calculate the gradient of the tangent (this will give you the initial rate of reaction):
Gradient = change in y-axis ÷ change in x-axis
Initial rate of reaction = 72 cm3 ÷ 20 s
Initial rate of reaction = 3.6 cm3 s-1
Rate: Temperature
- Enzymes have a specific optimum temperature – the temperature at which they catalyse a reaction at the maximum rate
- Lower temperatures either prevent reactions from proceeding or slow them down:
- Molecules move relatively slow
- Lower frequency of successful collisions between substrate molecules and active site of enzyme
- Less frequent enzyme-substrate complex formation
- Substrate and enzyme collide with less energy, making it less likely for bonds to be formed or broken (stopping the reaction from occurring)
- Higher temperatures speed up reactions:
- Molecules move more quickly
- Higher frequency successful collisions between substrate molecules and active site of enzyme
- More frequent enzyme-substrate complex formation
- Substrate and enzyme collide with more energy, making it more likely for bonds to be formed or broken (allowing the reaction to occur)
- However, as temperatures continue to increase, the rate at which an enzyme catalyses a reaction drops sharply, as the enzyme begins to denature:
- Bonds (eg. hydrogen bonds) holding the enzyme molecule in its precise shape start to break
- This causes the tertiary structure of the protein (ie. the enzyme) to change
- This permanently damages the active site, preventing the substrate from binding
- Denaturation has occurred if the substrate can no longer bind
- Very few human enzymes can function at temperatures above 50°C
- This is because humans maintain a body temperature of about 37°C, therefore even temperatures exceeding 40°C will cause the denaturation of enzymes
- High temperatures causes the hydrogen bonds between amino acids to break, changing the conformation of the enzyme

Limiting Factors Affecting Enzymes: pH
pH
- All enzymes have an optimum pH or a pH at which they operate best
- Enzymes are denatured at extremes of pH
- Hydrogen and ionic bonds hold the tertiary structure of the protein (ie. the enzyme) together
- Below and above the optimum pH of an enzyme, solutions with an excess of H+ ions (acidic solutions) and OH- ions (alkaline solutions) can cause these bonds to break
- This alters the shape of the active site, which means enzyme-substrate complexes form less easily
- Eventually, enzyme-substrate complexes can no longer form at all
- At this point, complete denaturation of the enzyme has occurred
- Where an enzyme functions can be an indicator of its optimal environment:
- Eg. pepsin is found in the stomach, an acidic environment at pH 2 (due to the presence of hydrochloric acid in the stomach’s gastric juice)
- Pepsin’s optimum pH, not surprisingly, is pH 2

When investigating the effect of pH on the rate of an enzyme-catalysed reaction, you can use buffer solutions to measure the rate of reaction at different pH values:
- Buffer solutions each have a specific pH
- Buffer solutions maintain this specific pH, even if the reaction taking place would otherwise cause the pH of the reaction mixture to change
- A measured volume of the buffer solution is added to the reaction mixture
- This same volume (of each buffer solution being used) should be added for each pH value that is being investigated
Calculating pH
- If the hydrogen ion (H+) concentration of a solution is known, the pH can be calculated using the equation:
pH = - log10 [H+] - You can find the ‘log’ function on your calculator (‘log’ is the same as ‘log10’ so don’t worry if your calculator doesn’t say ‘log10’)
Example: The hydrogen ion concentration of a solution is 1.6 x 10-4 mol dm-3. Find the pH of this solution.
The pH of the solution is:
pH = -log₁₀ [H⁺]
pH = -log₁₀ 1.6 x 10-4 = 3.796
pH = 3.8
Example: The hydrogen ion concentration of a solution of sodium hydroxide is 3.5 x 10-11 mol dm-3. Find the pH of this solution.
The pH of the solution is:
pH = -log₁₀ [H⁺]
pH = -log₁₀ 3.5 x 10-11 = 10.456
pH = 10.5
Example: Ethanoic acid (also known as acetic acid) is a weak acid produced by wood ants that they can spray at predators as a defence mechanism. The hydrogen ion concentration of a sample of ethanoic acid taken from some wood ants was 8.39 x 10-6 mol dm-3. Find the pH of the ethanoic acid produced by wood ants.
The pH of the solution is:
pH = -log₁₀ [H⁺]
pH = -log₁₀ 8.39 x 10-6 = 5.076
pH = 5.08
Limiting Factors Affecting Enzymes: Enzyme Concentration
Enzyme Concentration
- Enzyme concentration affects the rate of reaction
- The higher the enzyme concentration in a reaction mixture, the greater the number of active sites available and the greater the likelihood of enzyme-substrate complex formation
- As long as there is sufficient substrate available, the initial rate of reaction increases linearly with enzyme concentration
- If the amount of substrate is limited, at a certain point any further increase in enzyme concentration will not increase the reaction rate as the amount of substrate becomes a limiting factor

Limiting Factors Affecting Enzymes: Substrate Concentration
Substrate Concentration
- The greater the substrate concentration, the higher the rate of reaction:
- As the number of substrate molecules increases, the likelihood of enzyme-substrate complex formation increases
- If the enzyme concentration remains fixed but the amount of substrate is increased past a certain point, however, all available active sites eventually become saturated and any further increase in substrate concentration will not increase the reaction rate
- When the active sites of the enzymes are all full, any substrate molecules that are added have nowhere to bind in order to form an enzyme-substrate complex
- For this reason, in the graph below there is a linear increase in reaction rate as substrate is added, which then plateaus when all active sites become occupied

Limiting Factors Affecting Enzymes: Inhibitors
Enzyme Inhibitors
- An enzyme's activity can be reduced or stopped, temporarily, by a reversible inhibitor
- There are two types of reversible inhibitors:
- Competitive inhibitors have a similar shape to that of the substrate molecules and therefore compete with the substrate for the active site
- Non-competitive inhibitors bind to the enzyme at an alternative site, which alters the shape of the active site and therefore prevents the substrate from binding to it

Competitive and non-competitive inhibition
- Reversible inhibitors can act as regulators in metabolic pathways
- Metabolic reactions must be very tightly controlled and balanced, so that no single enzyme can ‘run wild’ and continuously and uncontrollably generate more and more of a particular product
- Metabolic reactions can be controlled by using the end-product of a particular sequence of metabolic reactions as a non-competitive, reversible inhibitor:
- As the enzyme converts substrate to product, the process is itself slowed down as the end-product of the reaction chain binds to an alternative site on the original enzyme, changing the shape of the active site and preventing the formation of further enzyme-substrate complexes
- The end-product can then detach from the enzyme and be used elsewhere, allowing the active site to reform and the enzyme to return to an active state
- This means that as product levels fall, the enzyme begins catalysing the reaction once again, in a continuous feedback loop
- This process is known as end-product inhibition

Inhibitor Concentration
- There are two types of inhibitors:
- Competitive inhibitors have a similar shape to that of the substrate molecules and therefore compete with the substrate for the active site
- Non-competitive inhibitors bind to the enzyme at an alternative site, which alters the shape of the active site and therefore prevents the substrate from binding to it
- Both types of inhibitors slow down or stop enzyme activity
- Increasing the concentration of an inhibitor, therefore, reduces the rate of reaction and eventually, if inhibitor concentration continues to be increased, the reaction will stop completely
- For competitive inhibitors, countering the increase in inhibitor concentration by increasing the substrate concentration can increase the rate of reaction once more (more substrate molecules mean they are more likely to collide with enzymes and form enzyme-substrate complexes)
- For non-competitive inhibitors, increasing the substrate concentration cannot increase the rate of reaction once more, as the shape of the active site of the enzyme remains changed and enzyme-substrate complexes are still unable to form

The effect of inhibitor concentration on the rate of an enzyme-catalysed reaction
Models & Functions of Enzyme Action
Models of enzyme action
- Scientists often use models to explain their observations from experiments
- As technology and research advances within a field new models can be developed and old ones disproven
- The lock and key model covered at GCSE was originally thought to be an accurate model of enzyme action
- It suggested that the rigid shape of the active site of the enzyme was a precise fit for the specific shape of the substrate
- New techniques have allowed scientists to discover that proteins are not rigid structures
- Experiments showed that multiple regions of an enzyme molecule moved in response to the environment
- Many of these movements were minimal but some of them were more significant
- The larger movements occurred when the substrate bound to the enzyme
- These findings led to the now widely accepted induced fit model
- Prior to binding, the substrate and active site and not completely complementary in shape
- When the substrate binds the active site alters shape and moulds around the substrate
- There is evidence to support the induced fit model:
- X-ray diffraction techniques allow for 3D pictures of molecules to be formed
- This technique was used to produce pictures of the enzyme hexokinase before and after it bound to its substrate glucose
- The images confirmed that the active site of the enzyme changed shape after the substrate bound

The lock-and-key hypothesis
The induced-fit hypothesis
Controlling Variables & Calculating Uncertainty
- Enzyme rate experiments are experiments that are carried out to determine the effect of changing a particular factor on the rate of a reaction that is catalysed by an enzyme
- Factors that can be changed include:
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
- The key thing with enzyme rate experiments is to ensure that only one of these variables is changed during a particular experiment
- This is known as the independent variable
- All other variables must be controlled (they must stay the same)
- These are known as the control variables
- For example, if investigating the effect of temperature on the rate of reaction, the pH, enzyme concentration and substrate concentration must be exactly the same (kept constant) each time you run the experiment (at each different temperature you are investigating)
- If these control variables are not kept constant, they could affect the results of the experiment
- This would make the results unreliable
Uncertainty
- Uncertainty is the amount of error your measurements might contain
- Results from experiments (including enzyme rate experiments) always contain some error (they are never perfect)
- There will always be a small degree of uncertainty in your readings or measurements
- This is often because the sensitivity of the apparatus being used is limited
- For example, you may want to measure reaction rate by measuring how much of a product is made in a given time period (e.g. using a gas syringe to measure the volume of oxygen produced from the breakdown of hydrogen peroxide by catalase)
- The gas syringe may only give readings to the nearest 1 cm3
- However, the real volume produced could be up to 0.05 cm3 smaller or larger
- It has an uncertainty value of ± 0.05 cm3
- A ‘±’ sign tells you the range in which the true value lies
- This range is called the margin of error
- For enzyme rate experiments, you may need to calculate the percentage error of your measurements
- As long as you know the uncertainty value of your measurements, the percentage error can be calculated using the following formula:
percentage error = (uncertainty value ÷ your measurement) x 100
- As long as you know the uncertainty value of your measurements, the percentage error can be calculated using the following formula:
Example 1: In an enzyme rate reaction involving the breakdown of hydrogen peroxide by catalase, 50 cm3 of oxygen was produced, with an uncertainty value of 0.05 cm3. Calculate the percentage error of this measurement.
Percentage error = (uncertainty value ÷ your measurement) x 100
Percentage error = (0.05 ÷ 50) x 100
Percentage error = 0.001 x 100
Percentage error = 0.1%
Example 2: In an enzyme rate experiment involving the breakdown of hydrogen peroxide by catalase, a student recorded that 10 cm3 of oxygen was produced in 5.245 seconds. The student measured this using a stopwatch that counted in milliseconds. Calculate the percentage error of the stopwatch measurements.
Step 1: Calculate the uncertainty value
- The stopwatch can measure to the nearest millisecond (0.001 second)
- This means the actual time taken could be up to 0.0005 seconds shorter or longer than this
- This means stopwatch measurements have an uncertainty value of ± 0.0005 s
Step 2: Calculate the percentage error of the student’s measurement of 5.245 seconds
- Percentage error = (uncertainty value ÷ your measurement) x 100
- Percentage error = (0.0005 ÷ 5.245) x 100
- Percentage error = 0.000095 x 100
- Percentage error = 0.0095% or 0.01%
|
122 videos|161 docs|138 tests
|

|
Explore Courses for Grade 12 exam
|

|










 Step 2: Find the tangent to the curve at 0 seconds (the start of the reaction)
Step 2: Find the tangent to the curve at 0 seconds (the start of the reaction)

























