Metallic Nitrosyls | Inorganic Chemistry PDF Download
Introuction
Metal nitrosyls are coordination compounds in which NO molecule is attached as NO+ ion to the metal atom or ion. In these compounds the attachment of NO+ ion to the metal atom or ion takes place through N atom. In these compounds NO+. In these compounds NO+ ion which is called nitrosonium or nitrosyl cation acts as a ligand. The coordination compounds of transition metals containing NO+ ion as ligand are metal (or metallic) nitrosyls. Examples of metal nitrosyls are given below:
(i) Metal Nitrosyl Carbonyls:
Important example of this type of coordination compounds are [Co- (NO+)(CO)3]0, [Fe2- (NO+)2(CO)2]0, [Mn3- (NO+)3(CO)]0, [Mn- (NO+)(CO)4]0, [V- (NO+)(CO)5]0 etc.
(ii) Metal nitrosyl halides:
These compounds are represented by [Fe- (NO+)2I]2, [Fe2- (NO+)2(CO)2]0, [Fe- (NO+)2I]0, [Fe2- (NO+)3Cl]0, [Co- (NO+)2X]0 (X = Cl, Br, I), [M- (NO+)2Cl2]0 (M = Mo or W).
(iii) Metal nitrosyl thio complexes:
These compounds are given by only Fe, Co and Ni. Examples are M+[Fe- (NO+)2S]- , M+[Co- (NO+)2S]- , M+[Ni- (NO+)2S]- (M = Na+, K+, NH4.
(iv) Metal nitrosyl cyano complexes:
Example of this type of complexes are [Mn+(NO+)(CN)5]2- , [Fe+(NO+)(CN)5]2- , [Mn+(NO+)(CN)5]3- , [Mo+(NO+)(CN)5]4-. Among these complexes, pentacyano nitrosyl ferrate(II) ion, [Fe+(NO+)(CN)5]2- is the most important.
(v) Miscellaneous metal nitrosyl complexes:
Nitrosyl complexes like [Co+(NO+)(NH3)5]2+, [Co+(NO+)(NO2)5]3- , [Fe+(NO+)]2+, [Ru2+(NO+)(NH3)4Cl]2+, [Ru2+(NO+)Cl5]2- , [Fe2- (NO+)2(PR3)3]0 etc. Among these complexes, [Fe+(NO+)]2+ is the most important.
Preparation
(i) Metal nitrosyl carbonyls can be obtained by the action of NO on metal carbonyls, e.g.,
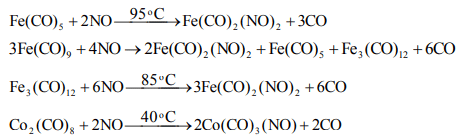
Properties of metal nitrosyl carbonyls:
(i) Substitution reactions: In metal carbonyl nitrosyls, NO+ ions are more firmly attached with the metal ion than the CO groups. It is for this reason that when metal carbonyl nitrosyls are treated with ligands like PR3, CNR, phen etc., it is only CO groups that are replaced by these ligands, e.g.,
Fe(CO)2(NO)2+2L(L=PRa, CNR) →Fe(L)2(NO)2+2CO
Fe(CO)2(NO)2+phen →Fe(phen)(NO)2+2CO
(ii) Action of halogens: Many metal carbonyl nitrosyls, when treated with halogens, are converted into metal nitrosyl halides, eg.,
2[Fe(CO)2(NO)2]+I2 → [Fe(NO)2I]2+4CO
Properties of metal nitrosyl halides:
(i) Metal nitrosyl halides react with other ligands to form mono-nuclear complexes, e.g., [Fe(NO)2X]2 + 2L→ 2[Fe(NO)2XL]
(ii) Iron nitrosyl halide, [Fe(NO)2I]2 reacts with K2S and CH3CI to form dark red compounds which have the composition, K2[Fe(NO)2S]2 and [Fe(NO)2(SCH3)]2 and are called Roussin's salts. In these compounds, Fe is in -1 oxidation state.
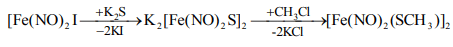
Some Metallic Nitrosyls
Now let us make study of sodium nitroprusside, Na2[Fe2+(CN)5(NO+)] and nitroso ferrous sulphate, FeSO4NO or [Fe+(NO+)]SO4 which are important metallic nitrosyls.
1) Sodium nitroprusside, Na2[Fe2+(CN)5(NO+)]:
Preparation: It is prepared
(i) By the action of NaNO3 on Na4[Fe2+(CN)6]
Na4[Fe2+(CN)6] + NaNO2 + H2O → Na2[Fe2+(CN)5(NO+)] + 2NaOH + NaCN
(ii) by passing nitric oxide (NO) into acidified solution of Na4[Fe(CN)6]. 2Na4[Fe(CN)6] + H2SO4 + 3NO → 2Na2[Fe(NO)(CN)5] + 2NaCN + Na2SO4 + l/2 N2 + H2O
Properties:
(i) Sodium nitroprusside forms beautiful ruby red rhombic crystals which are soluble in water.
(ii) When freshly prepared sodium nitroprusside is added to a solution containing sulphide ion (i.e. Na2S but not H2S), a purple or violet colour is produced. the production of this colour is due to the formation of Na4[Fe2+(CN)5(NO+)(S2-)]. The production of this purple or violet colour is used to confirm the presence of S2- ion in a given mixture.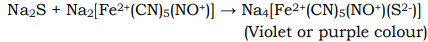
(iii) Alkali sulphites give a rose red colour due to the formation of Na4[Fe(CN)5(NO)(SO3)]. This reaction can be used to distinguish sulphites from thiosulphates which do not show this reaction.
Na2SO3 + Na2[Fe(CN)5(NO)] → Na4[Fe(CN)5(NO)(SO3)]
(iv) With silver nitrate a flesh coloured Ag2[Fe(CN)5(NO)] is produced.
2AgNO3+Na2[Fe(CN)5(NO)] → Ag2[Fe(CN)5(NO)]+2NaNO3
(v) Aldehydes and ketones containing CH3-CO-R group give deep red colour with sodium nitroprusside and excess of NaOH.
(vi) It is converted into sodium ferrocyanide, Na4[Fe(CN)6] on treatment with an alkali.
6Na2[Fe(CN)5(NO)] + 14NaOH → 5Na4[Fe(CN)6] + Fe(OH)2 + 6NaNO3 + 6H2O According to another view NO+ groups present in nitroprusside is oxidized to NO2 and thus a nitro complex is obtained.
Na2[Fe(CN)5(NO)] + 2NaOH → Na4[Fe(CN)5(NO2)] + H2O
(vii) [Fe(CN)5(NO)]2- ion has diamagnetic character. Its diamagnetic character confirms the fact that NO is present as NO+ ion in this complex ion.
Structure:
[Fe(CN)5(NO)]2- was formerly supposed to contain Fe(+3) ion but Pauling in 1931 and Sidgwick in 1934 suggested that the odd electron of NO group enters the valence-shell of Fe (+3) ion making Fe in +2 oxidation state. Thus NO radical acquires one positive charge and gets coordinated to Fe(+2) ion as NO+ radical. This view is supported by the fact that Na2[Fe(CN)5(NO)] is diamagnetic where as K3[Fe(CN)6] is paramagnetic. Thus in [Fe(CN)5(NO)]2- there are total three positive charges (Fe = +2, NO = +1] and five negative charges due to the presence of five CN groups. Hence total charges acquired by [Fe(CN)5(NO)] is -2. In other words, the formula of sodium nitroprusside is Na2[Fe2+(CN)5 (NO+)]. [Fe2+(CN)5(NO+)]2- has octahedral structure with Fe2+ ion located at the centre of the octahedron.
Uses:
It is use as a reagent in qualitative analysis for the detection of sulphides, sulphites, aldehydes and ketones containing CH3-CO-R group.
2) Nitroso ferrous sulphate, FeSO4NO or [Fe+ (NO+)]SO4:
When, to the aqueous solution of a metallic nitrate (say NaNO3) is added freshly prepared solution of FeSO4 and a few drops of conc. H2SO4 along the sides of the test tube, a brown ring of nitroso ferrous sulphate, [Fe+(NO+)]SO4 is obtained at the junction of the two liquids in the test tube. The formation of nitroso ferrous sulphate takes place through the following equations:
(a) 6NaNO3 + H2SO4 → NaHSO3 + HNO3
[or NO3- + H+ → HNO3]
(b) 6FeSO4 + 2HNO3 + 3H2SO4 → 3Fe2(SO4)3 + 2NO + 4H2O
[or 3Fe2+ + NO3+ + 4H+ → 3Fe3+ + NO + 2H2O]
(c) FeSO4 + NO → FeSO4NO or [Fe+(NO+)]2+SO42-
[or Fe2+ + NO → [Fe+(NO+)]2+]
In aqueous solution [Fe+(NO+)]2+ ion is better expressed as [Fe(NO)(H2O)5]2+. It is a paramagnetic substance corresponding to the presence of three unpaired electrons, since solution magnetic measurements give 3.90 B.M. as the value of its magnetic moment. This value supports the fact that Fe is in +1 oxidation state in this complex ion i.e. it is a high spin complex of Fe (+1) (3d7 system) with NO+. The complex has N—O stretching frequency at 1745 cm-1 which indicates the presence of strong π-bonding and the intense brown colour strongly suggests Fe+—NO+ charge transfer.
The formation of [Fe+(NO+)]2+ ion has been utilized in the detection of NO3- ion in a given inorganic salt.
Structure and nature of M—NO bonding in nitrosyls:
According to Coulson the molecular orbital configuration of NO molecule is (sp)2O, (σspb) 2,(πyb)2 = (πzb)2, (sp)2N, (πy*)1 = πz*)0, (σsp*)0 [in getting this configuration x-axis has been assumed to be the molecular axis and sp hybrid orbital have been obtained by the combination of 2s and 2px orbital]. Now when NO molecule coordinates with metal atom to form metallic nitrosyls, the single electron present in πy* molecular orbital is transferred to metal atom M so that NO molecule is converted into NO+ cation (called nitrosonium or nitrosyl cation) and M atom becomes M- ion. Each of the atoms viz. N and O in NO+ ion contains one lone pair of electrons in (sp)O and (sp)N hybrid orbital respectively. Now since NO+ ion is iso-electronic with CO molecule, this ion co-ordinates with M- ion as a two-electron donor in metal nitrosyls in the same way as CO co-ordinates to M atom in metal carbonyls. Note that, NO molecule is a three-electron-donor. Since O-atom is more electronegative than N-atom, it is N-atom of NO+ ion which coordinates to M- ion metal nitrosyls. In other wards we say that coordination of NO+ ion to M- ion metal nitrosyl takes place through the lone pair residing in (sp)N hybrid orbital on N-atom. The coordinate bond formed in metal nitrosyls can be shown as M- ←NO+. Thus, we see that the nature of bonding between NO+ and Min nitrosyls is the same as that between CO molecule and M atom in carbonyls.
On the basis of molecular orbital theory, the hybrid orbital on N-atom containing a lone pair [i.e. (sp)2N lone pair] overlaps with a suitable vacant hybrid orbital on M- ion (sp3 in tetrahedral or d2sp3 in octahedral case) to form ON+ → M- σ-bond. Now the empty πx* or πy* molecular orbital can overlap with the filled d-orbitals (dxy, dyz, dzx orbitals in octahedral case and dx2-y2 and dz2 orbitals in tetrahedral case) to form M- ←NO+ π-bond. This type of overlap transfers some charge from M- ion to NO+ ion. Structural studies have shown that the M—N bond in nitrosyls is extremely short (= 1.57 to 1.67A0) indicating substantial double bond character.
Effective atomic number (EAN) rule as ap| metallic nitrosyls:
Metallic nitrosyls also obey the EAN shown below for certain nitrosyls. In these cases, NO (assumed to be a unipositive ion, NO+ and hence acts as an electron donor. Metal atoms are, therefore, in negative o state.
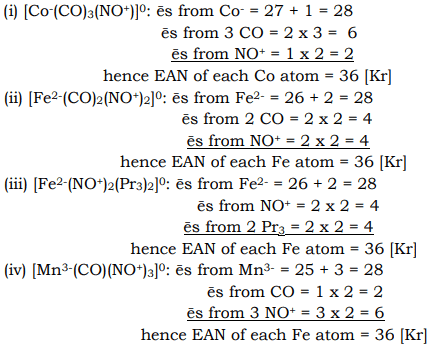
|
50 videos|92 docs|41 tests
|
FAQs on Metallic Nitrosyls - Inorganic Chemistry
| 1. What are metallic nitrosyls and how do they form? |  |
| 2. What are the properties of metallic nitrosyls? |  |
| 3. How are metallic nitrosyls used in industrial applications? |  |
| 4. Are metallic nitrosyls toxic? |  |
| 5. Can metallic nitrosyls be found in nature? |  |





















