Mechanical Engineering Exam > Mechanical Engineering Notes > Thermodynamics > Mind Map: Basic Concepts & Zeroth Law of Thermodynamics
Mind Map: Basic Concepts & Zeroth Law of Thermodynamics | Thermodynamics - Mechanical Engineering PDF Download
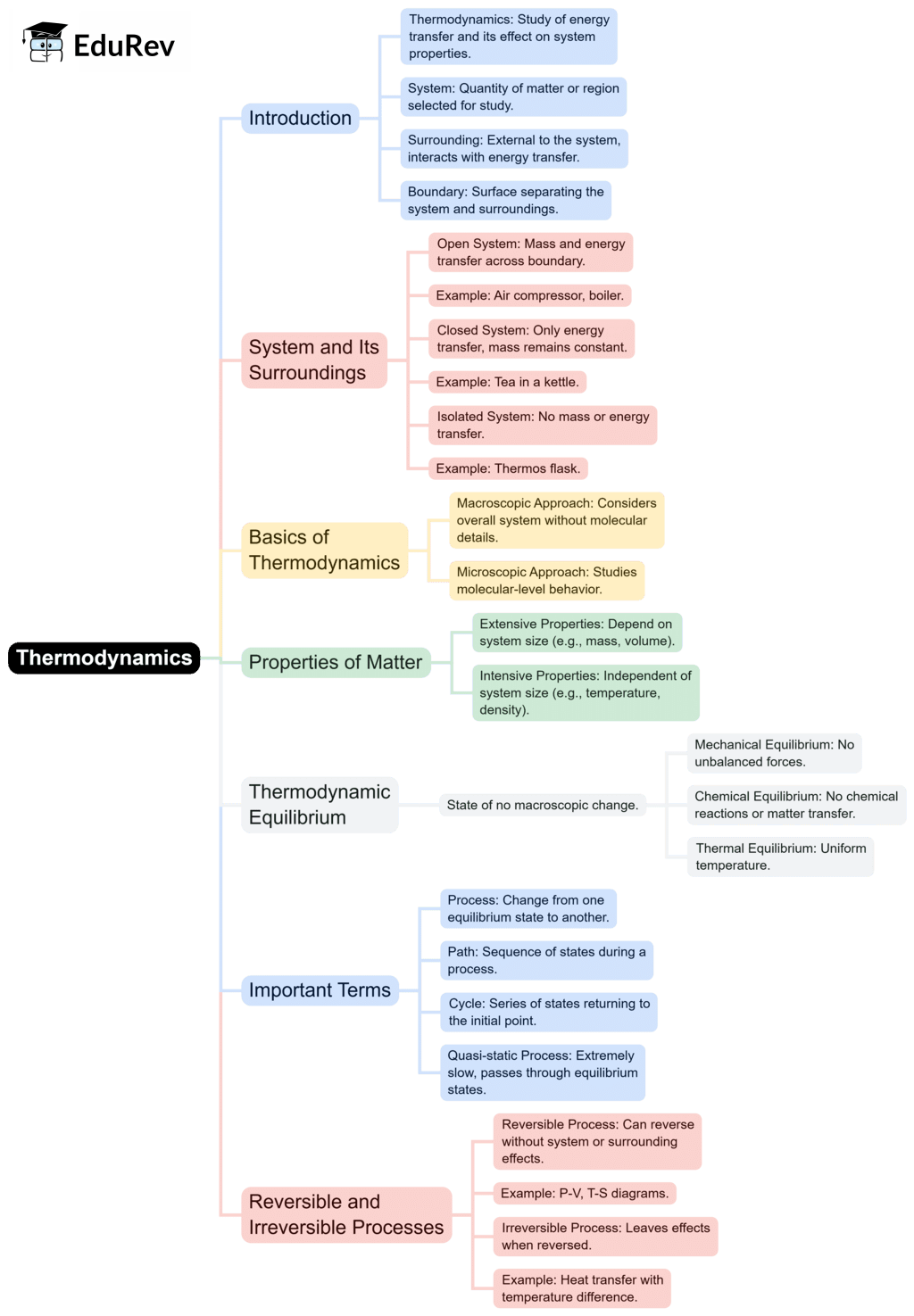
The document Mind Map: Basic Concepts & Zeroth Law of Thermodynamics | Thermodynamics - Mechanical Engineering is a part of the Mechanical Engineering Course Thermodynamics.
All you need of Mechanical Engineering at this link: Mechanical Engineering
|
29 videos|150 docs|36 tests
|
FAQs on Mind Map: Basic Concepts & Zeroth Law of Thermodynamics - Thermodynamics - Mechanical Engineering
| 1. What is the Zeroth Law of Thermodynamics and why is it important? |  |
Ans. The Zeroth Law of Thermodynamics states that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law is fundamental as it establishes the concept of temperature and allows for the comparison of temperatures between different systems. It is important because it provides a basis for the definition of temperature and enables the development of thermometers.
| 2. How does the Zeroth Law of Thermodynamics relate to temperature measurement? |  |
Ans. The Zeroth Law of Thermodynamics is essential for temperature measurement because it implies that if two objects are at the same temperature as a third object, they must be at the same temperature as each other. This principle allows thermometers to reliably measure temperature by ensuring that the thermometer itself is in thermal equilibrium with the substance being measured, thus providing accurate readings.
| 3. Can you give an example of the Zeroth Law of Thermodynamics in practical applications? |  |
Ans. A practical example of the Zeroth Law of Thermodynamics is the functioning of a mercury thermometer. When the thermometer is placed in a fluid, it reaches thermal equilibrium with the fluid. According to the Zeroth Law, if the thermometer and the fluid are both in equilibrium with the surrounding environment, then the temperature indicated by the thermometer accurately reflects the temperature of the fluid.
| 4. What are the implications of the Zeroth Law of Thermodynamics in engineering? |  |
Ans. The implications of the Zeroth Law of Thermodynamics in engineering include the ability to design and use temperature sensors, control systems, and thermal management solutions. Understanding this law helps engineers ensure that equipment operates within desired temperature ranges and enhances the safety and efficiency of thermal systems in various applications, such as HVAC, automotive, and aerospace engineering.
| 5. How does the Zeroth Law of Thermodynamics connect to the first three laws of thermodynamics? |  |
Ans. The Zeroth Law of Thermodynamics serves as a foundation for the first three laws of thermodynamics by establishing the concept of thermal equilibrium and temperature. The first law (conservation of energy), the second law (entropy), and the third law (absolute zero) build upon the understanding of temperature and thermal interactions defined by the Zeroth Law. Together, these laws form the core principles governing thermodynamic processes and energy exchanges.
Related Searches
















