SSS 1 Exam > SSS 1 Notes > Chemistry for SSS 1 > Mind Map: Elements, Compounds & Mixtures
Mind Map: Elements, Compounds & Mixtures | Chemistry for SSS 1 PDF Download
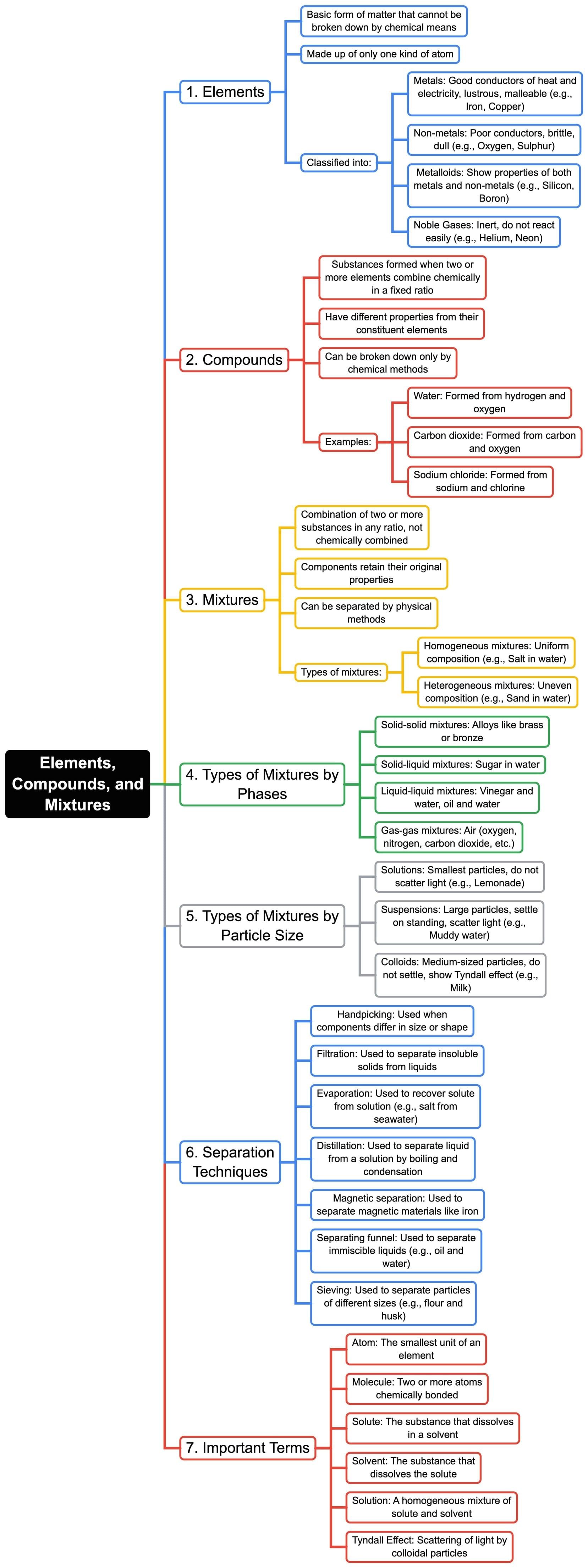
The document Mind Map: Elements, Compounds & Mixtures | Chemistry for SSS 1 is a part of the SSS 1 Course Chemistry for SSS 1.
All you need of SSS 1 at this link: SSS 1
|
19 videos|100 docs|23 tests
|
FAQs on Mind Map: Elements, Compounds & Mixtures - Chemistry for SSS 1
| 1. What are the main differences between elements, compounds, and mixtures? |  |
Ans.Elements are pure substances that consist of only one type of atom, such as hydrogen (H) or oxygen (O). Compounds are substances formed when two or more different elements chemically combine in a fixed ratio, like water (H₂O) or carbon dioxide (CO₂). Mixtures, on the other hand, are combinations of two or more substances that retain their individual properties and can be separated physically, like air or a salad.
| 2. Can you provide examples of elements, compounds, and mixtures? |  |
Ans.Examples of elements include gold (Au), iron (Fe), and helium (He). For compounds, common examples are sodium chloride (NaCl) and methane (CH₄). Mixtures can be represented by examples such as saltwater, which is a mixture of salt (NaCl) and water (H₂O), or a concrete mixture, which combines cement, water, and aggregates.
| 3. How can mixtures be separated into their components? |  |
Ans.Mixtures can be separated using various physical methods based on the properties of the components. Techniques include filtration, where solid particles are separated from liquids; distillation, which separates liquids based on boiling points; and chromatography, which separates substances based on their movement through a medium. Each method takes advantage of differences in physical properties.
| 4. What is meant by a homogeneous mixture and a heterogeneous mixture? |  |
Ans.A homogeneous mixture is one where the components are uniformly distributed and cannot be easily distinguished, such as vinegar or air. In contrast, a heterogeneous mixture is one where the different components are visible and can be easily separated, like a salad or a rock mixture. The uniformity of distribution is key to distinguishing between these two types of mixtures.
| 5. Why are compounds considered to have different properties than the elements that compose them? |  |
Ans.Compounds exhibit distinct properties that are different from the individual elements due to the chemical reactions that occur when elements combine. For instance, sodium (Na) is a highly reactive metal, and chlorine (Cl) is a toxic gas; however, when they combine to form sodium chloride (NaCl), they create a stable and safe substance known as table salt. This transformation results in properties that differ significantly from those of the constituent elements.
Related Searches





















