Class 6 Exam > Class 6 Notes > Science Olympiad Class 6 > Mind Map: A Journey through States of Water
Mind Map: A Journey through States of Water | Science Olympiad Class 6 PDF Download
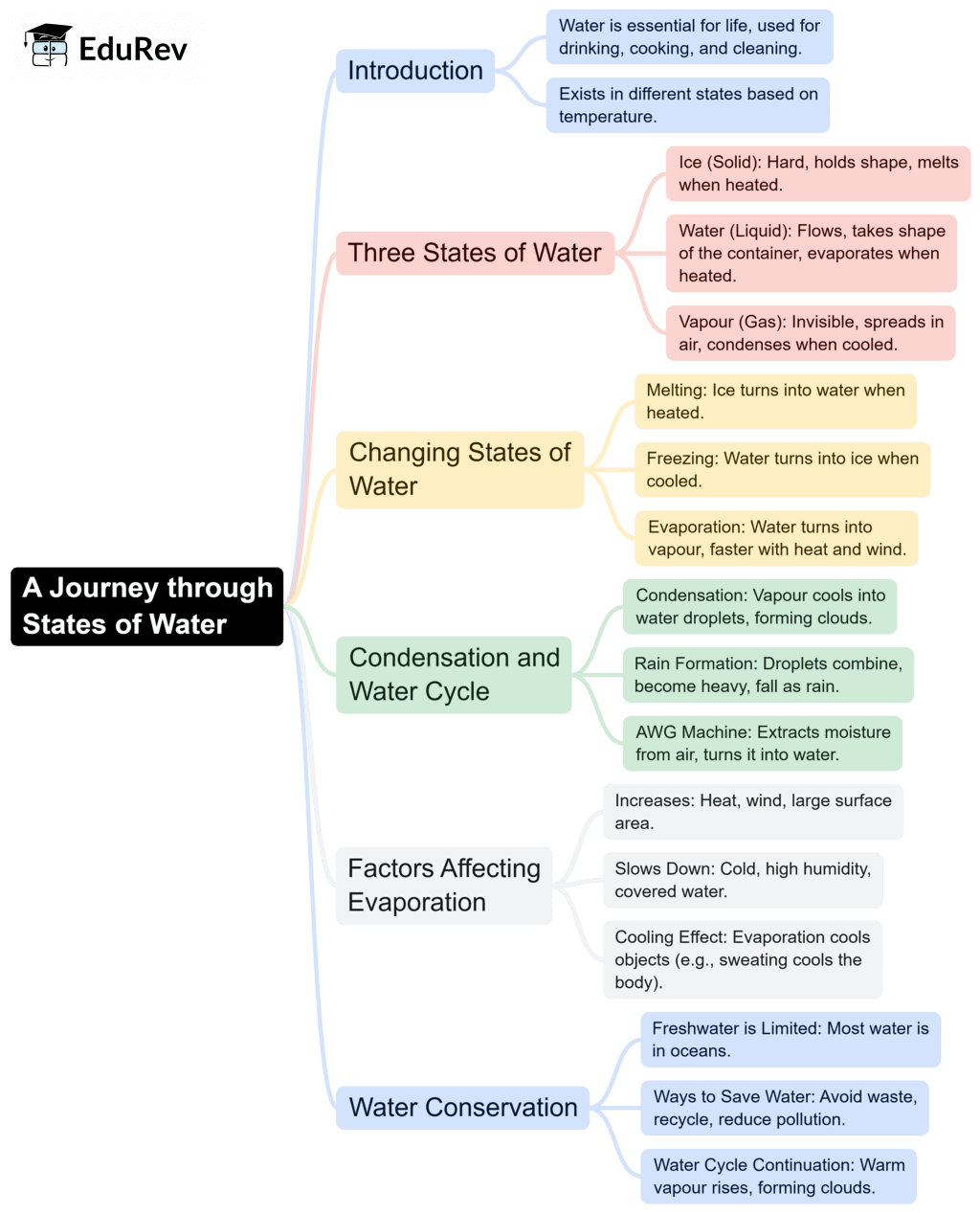
The document Mind Map: A Journey through States of Water | Science Olympiad Class 6 is a part of the Class 6 Course Science Olympiad Class 6.
All you need of Class 6 at this link: Class 6
|
48 videos|116 docs|89 tests
|
FAQs on Mind Map: A Journey through States of Water - Science Olympiad Class 6
| 1. What are the three states of water, and how do they change from one state to another? |  |
Ans. The three states of water are solid (ice), liquid (water), and gas (water vapor). Water changes from solid to liquid when it is heated, a process called melting. Conversely, when water is cooled, it freezes to become solid. Liquid water can turn into gas through evaporation when heated, and gas can condense back into liquid when cooled.
| 2. What is the significance of the water cycle in understanding the states of water? |  |
Ans. The water cycle is a continuous process that describes how water moves through different states and locations on Earth. It includes processes such as evaporation, condensation, precipitation, and runoff. Understanding the water cycle helps us see how water changes states and is vital for sustaining life, regulating climate, and supporting ecosystems.
| 3. How does temperature affect the state of water? |  |
Ans. Temperature plays a crucial role in determining the state of water. At 0°C, water freezes to become ice (solid), and at 100°C, it boils to become water vapor (gas). Between these temperatures, water exists as a liquid. Changes in temperature can lead to phase transitions, such as melting, freezing, boiling, and condensation.
| 4. Can you explain the concept of density in relation to the states of water? |  |
Ans. Density is the mass of a substance per unit volume. In the case of water, its solid state (ice) is less dense than its liquid state, which is why ice floats on water. This unique property is important for aquatic life, as it insulates the water beneath the ice, allowing organisms to survive in cold conditions.
| 5. What role do impurities play in the freezing and boiling points of water? |  |
Ans. Impurities in water, such as salt or sugar, can alter its freezing and boiling points. The presence of impurities raises the boiling point and lowers the freezing point of water, a phenomenon known as boiling point elevation and freezing point depression. This is why seawater freezes at a lower temperature than pure water and why salt is used to melt ice on roads.
Related Searches
















