NCERT Based Activity: Atoms and Molecules | Science Class 9 PDF Download
Activity 3.1: Law of Conservation of Mass
Description:
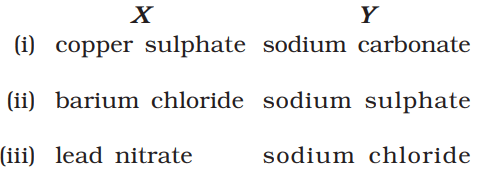
- Take one of the following sets of chemicals (X and Y):
- Copper sulphate (X) and sodium carbonate (Y)
- Barium chloride (X) and sodium sulphate (Y)
- Lead nitrate (X) and sodium chloride (Y)
- Prepare separately a 5% solution of any one pair of substances listed under X and Y, each in 10 mL of water.
- Take a little amount of solution Y in a conical flask and some solution of X in an ignition tube.
- Hang the ignition tube in the flask carefully, ensuring the solutions do not mix. Put a cork on the flask (see Fig. 3.1).
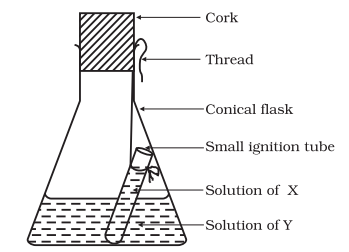 Fig. 3.1: Ignition tube containing solution of X, dipped in a conical flask containing solution of Y
Fig. 3.1: Ignition tube containing solution of X, dipped in a conical flask containing solution of Y - Weigh the flask with its contents carefully.
- Tilt and swirl the flask to mix solutions X and Y.
- Weigh again.
Q1: What happens in the reaction flask?
Ans: When the solutions of X and Y are mixed, a chemical reaction occurs, often forming a precipitate. For example:
- Copper sulphate + sodium carbonate → copper carbonate (precipitate) + sodium sulphate.
- Barium chloride + sodium sulphate → barium sulphate (precipitate) + sodium chloride.
- Lead nitrate + sodium chloride → lead chloride (precipitate) + sodium nitrate.
- A visible change, such as the formation of a solid (precipitate) or colour change, is observed.
Q2: Do you think a chemical reaction has taken place?
Ans: Yes, a chemical reaction has occurred, as evidenced by the formation of a precipitate or other visible changes, indicating the formation of new substances.
Q3: Why should we put a cork on the mouth of the flask?
Ans: The cork prevents any gaseous products or volatile substances from escaping, ensuring that the total mass of the system (reactants and products) remains unchanged during the reaction.
Q4: Does the mass of the flask and its contents change?
Ans: No, the mass of the flask and its contents does not change after mixing. The weight before and after the reaction is the same.
Explanation: The activity demonstrates the Law of Conservation of Mass, which states that mass is neither created nor destroyed in a chemical reaction. The total mass of the reactants (solutions X and Y) equals the total mass of the products (precipitate and remaining solution). The cork ensures no mass is lost as gas, and the formation of a precipitate confirms a chemical reaction. For example, in the reaction of copper sulphate and sodium carbonate, the precipitate (copper carbonate) forms, but the total mass remains constant, supporting the law.
Activity 3.2: Law of Definite Proportions
Description:
Refer to Table 3.4 for the ratio by mass of atoms present in molecules and Table 3.2 for atomic masses of elements.
Find the ratio by number of atoms of elements in the molecules of compounds given in Table 3.4 (water, ammonia, carbon dioxide).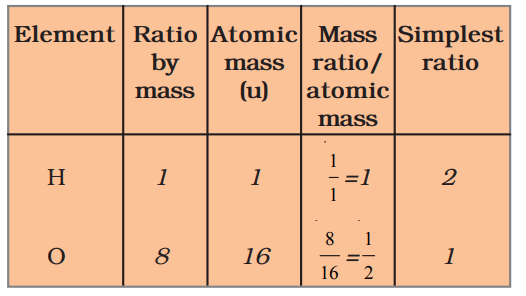
Example provided for water:
- Ratio by mass (H:O = 1:8)
- Atomic mass: H = 1 u, O = 16 u
- Mass ratio / atomic mass: H = 1/1 = 1, O = 8/16 = 1/2
- Simplest ratio: H:O = 2:1 (after multiplying by 2 to get whole numbers)
Ans: To calculate the ratio by number of atoms for the compounds listed in Table 3.4: water (H₂O), ammonia (NH₃), and carbon dioxide (CO₂).
Calculations:
Water (H₂O):
- Given: Ratio by mass (H:O = 1:8), Atomic mass (H = 1 u, O = 16 u)
- Mass ratio / atomic mass:
H: 1 / 1 = 1
O: 8 / 16 = 0.5 - Simplest ratio: Multiply by 2 to get whole numbers: H:O = 1 × 2 : 0.5 × 2 = 2:1
- Result: The ratio by number of atoms for water is H:O = 2:1.
Ammonia (NH₃):
- Given: Ratio by mass (N:H = 14:3), Atomic mass (N = 14 u, H = 1 u)
- Mass ratio / atomic mass:
N: 14 / 14 = 1
H: 3 / 1 = 3 - Simplest ratio: N:H = 1:3
- Result: The ratio by number of atoms for ammonia is N:H = 1:3.
Carbon Dioxide (CO₂):
- Given: Ratio by mass (C:O = 3:8), Atomic mass (C = 12 u, O = 16 u)
- Mass ratio / atomic mass:
- C: 3 / 12 = 0.25
- O: 8 / 16 = 0.5
- Simplest ratio: Multiply by 4 to get whole numbers: C:O = 0.25 × 4 : 0.5 × 4 = 1:2
- Result: The ratio by number of atoms for carbon dioxide is C:O = 1:2.
- Explanation: The ratio by number of atoms is calculated by dividing the mass ratio of each element in the compound by its atomic mass, then simplifying to the smallest whole-number ratio. This reflects the number of atoms of each element in the molecule, consistent with the molecular formula (H₂O, NH₃, CO₂). The activity illustrates the Law of Definite Proportions, as the ratio of atoms in each compound is fixed, and it aligns with Dalton’s postulate that atoms combine in small whole-number ratios to form compounds.
Group Activity: Play a Game for Writing Formulae
Description:
Example 1: Make playcards with symbols and valencies of elements separately. Each student holds two placards: one with the symbol in the right hand and one with the valency in the left hand. Students criss-cross their valencies to form the formula of a compound while keeping symbols in place.
Example 2: Use empty blister packs of medicines, cut into groups according to the valency of the element. Make formulae by fixing one type of ion into another.
- Example: Sodium sulphate (Na₂SO₄) – 2 sodium ions (Na⁺, valency 1) fixed on one sulphate ion (SO₄²⁻, valency 2).
- Task: Write the formula of sodium phosphate.
Ans: Formula for Sodium Phosphate:
Ions involved:
- Sodium: Na⁺ (valency 1, from Table 3.6)
- Phosphate: PO₄³⁻ (valency 3, from Table 3.6)
Criss-cross method:
- Sodium valency (1) becomes the subscript for phosphate.
- Phosphate valency (3) becomes the subscript for sodium.
- Formula: Na₃(PO₄) or simply Na₃PO₄ (brackets are not needed for a single polyatomic ion).
Explanation using blister pack model:
- Sodium ion (Na⁺) has a valency of 1, represented by one “slot” in the blister pack.
- Phosphate ion (PO₄³⁻) has a valency of 3, represented by three “slots” in the blister pack.
- To balance the charges, three sodium ions (each with one positive charge) are needed to combine with one phosphate ion (with three negative charges).
- Thus, the formula is Na₃PO₄, where three Na⁺ ions fit into the three slots of one PO₄³⁻ ion.
General Explanation: The activity teaches students to write chemical formulae by applying the concept of valency and the criss-cross method. The placard game visually represents how atoms combine based on their valencies, while the blister pack model simulates the “locking” of ions to form neutral compounds. For sodium phosphate, the formula Na₃PO₄ reflects the combination of three sodium ions with one phosphate ion to achieve charge neutrality, aligning with the rules for writing chemical formulae.
|
84 videos|478 docs|59 tests
|
FAQs on NCERT Based Activity: Atoms and Molecules - Science Class 9
| 1. What are the basic rules for writing chemical formulae? |  |
| 2. How do you determine the valency of an element when writing a formula? |  |
| 3. What is the significance of using subscripts in chemical formulae? |  |
| 4. Can you explain the difference between empirical and molecular formulae? |  |
| 5. What strategies can be used to help students learn to write chemical formulae effectively? |  |
















