NCERT Exemplar: Haloalkanes and Haloarenes | Chemistry for JEE Main & Advanced PDF Download
MULTIPLE CHOICE QUESTIONS (TYPE - I)
Q.1. The order of reactivity of following alcohols with halogen acids is ________.
(A) CH3CH2—CH2—OH
(B) 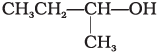
(C)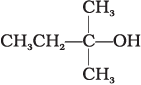
(i) (A) > (B) > (C)
(ii) (C) > (B) > (A)
(iii) (B) > (A) > (C)
(iv) (A) > (C) > (B)
Ans. (ii)
Solution.
The reactivity of alcohols depends on the type of alcohol (ie.primary / secondary / tertiary). It is due to the presence of nature of carbocation present in its structure
Higher the stability of carbocation, higher will be the possibilities of attack of X− ion to the carbocation. The order of reactivity of alcohols with a given haloacid is acid is 3º >2º >1º.
So, the correct option is (ii).
Q.2. Which of the following alcohols will yield the corresponding alkyl chloride on reaction with concentrated HCl at room temperature?
(i) CH3CH2—CH2—OH
(ii)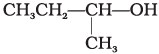
(iii) 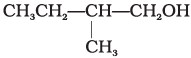
(iv) 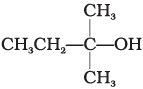
Ans. (iv)
Solution.
When alcohols are treated with conc. HCl at room temperature than alkyl chloride is formed. This reaction follows SN1 mechanism SN1 mechanism completes in two steps. In first step, a carbocation is formed and is attacked by nucleophile in second step.
The attack of nucleophile to the carbocation is possible only if the carbocation is stable. Compound present in option (iv) will give tertiary carbocation in step 1. Tertiary carbocation is the most stable so it is further attacked by Cl− nucleophile as follows.
Step I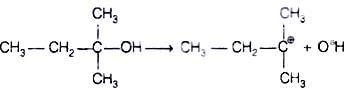 Step II
Step II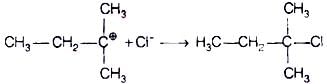
Q.3. Identify the compound Y in the following reaction.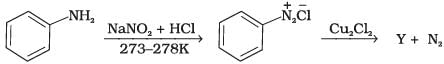
(i)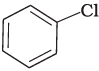
(ii)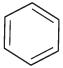
(iii)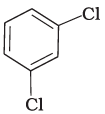
(iv)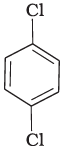
Ans. (i)
Solution.
When a primary aromatic amine is dissolved or suspended in cold aqueous mineral acid and treated with sodium nitrite, a diazonium salt is formed. When this freshly prepared diazonium salt is mixed with cuprous chloride, then diazonium group is replaced by Cl atom. Then chlorobenzene is formed which is y in this reaction.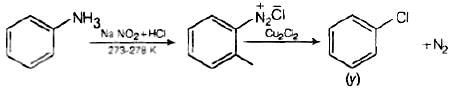
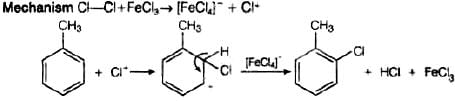
Q.4. Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is
(i) Electrophilic elimination reaction
(ii) Electrophilic substitution reaction
(iii) Free radical addition reaction
(iv) Nucleophilic substitution reaction
Ans. (ii)
Solution.
Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is electrophilic substitution reaction.
It has the following mechanism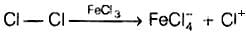
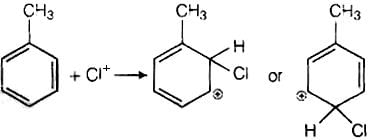
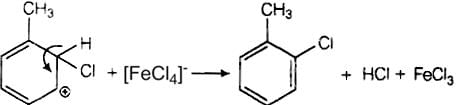 In this mechanism, electrophile Cl+ attacks to electron rich benzene ring and replaces hydrogen. So, the reaction is electrophilic substitution reaction.
In this mechanism, electrophile Cl+ attacks to electron rich benzene ring and replaces hydrogen. So, the reaction is electrophilic substitution reaction.
Q.5. Which of the following is halogen exchange reaction?
(i) RX + NaI → RI + NaX
(ii)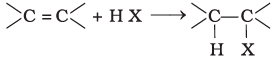
(iii)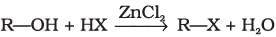
(iv)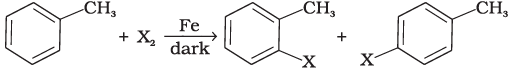
Ans. (i)
Solution.
Halogen exchange reactions are those reactions in which one halide replaces another. In option (i) halogen (−X) is replaced by iodine. This reaction is named as Finkelstein reaction.
In option (ii), there is the addition of hydrogen halide on alkene.
In option (iii), halogen replaces alcoholic group.
While in option (iv) halogen replaces the hydrogen of benzene line.
Q.6. Which reagent will you use for the following reaction?
CH3CH2CH2CH3 → CH3CH2CH2CH2Cl + CH3CH2CHClCH3
(i) Cl2/UV light
(ii) NaCl + H2SO4
(iii) Cl2 gas in dark
(iv) Cl2 gas in the presence of iron in dark
Ans. (i)
Solution.
The given reaction is a substitution reaction. It involves the replacement of 1° or 2° hydrogen of alkanes by chlorine. It occurs in presence of ultraviolet light or at high temperature.
The chlorination does not occur at room temperature in absence of light. In this reaction, light is absorbed by the chlorine molecule and activated chlorine initiates the reaction as follows
Step 1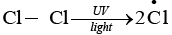
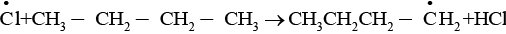
Step 2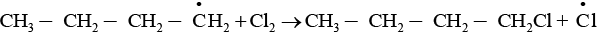
Step 3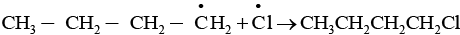
So, option (i) is the correct
Q.7. Arrange the following compounds in the increasing order of their densities.
(a)
(b)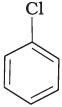
(c)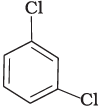
(d)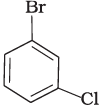
(i) (a) < (b) < (c) < (d)
(ii) (a) < (c) < (d) < (b)
(iii) (d) < (c) < (b) < (a)
(iv) (b) < (d) < (c) < (a)
Ans. (i)
Solution.
Density is directly related to molecular mass. Higher the molecular mass, higher will be the density of the compound. Among the four given compounds, the order of molecular mass is benzene < chlorobenzene < dichlorobenzene < bromochlorobenzene
Therefore, the increasing order of their densities is same as above.
Hence, option (i) is correct.
Q.8. Arrange the following compounds in increasing order of their boiling points.
(a)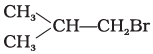
(b) CH3CH2CH2CH2Br
(c)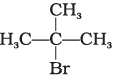
(i) (b) < (a) < (c)
(ii) (a) < (b) < (c)
(iii) (c) < (b) < (d)
(iv) (c) < (b) < (a)
Ans. (iii)
Solution.
Boiling point of a compound depends upon the surface area. Higher the surface area higher will be the boiling point of a compound. Surface area decreases with increase in branching in a molecule, so its boiling point will also decrease.
Hence, option (iii) is correct.
Q.9. In which of the following molecules carbon atom marked with asterisk (*) is asymmetric?
(a)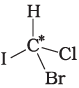
(b)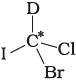
(c)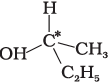
(d)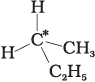
(i) (a), (b), (c), (d)
(ii) (a), (b), (c)
(iii) (b), (c), (d)
(iv) (a), (c), (d)
Ans. (ii)
Solution.
Asymmetric/chiral carbon atom is that in which all of its four valencies with four different groups or atoms. In compound (iv), carbon satisfies two of its valencies with two hydrogen atoms i,e. similar atom.
So, it is not an asymmetric carbon atom while rest of the three molecules have asymmetric carbon as each carbon has satisfied all four valencies with four different groups or atoms.
So, the correct option is (ii).
Q.10. Which of the following structures is enantiomeric with the molecule (A) given below :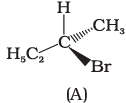
(i)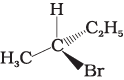
(ii) 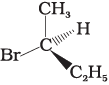
(iii)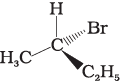
(iv)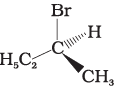
Ans. (i)
Solution.
The stereoisomers related to each other as non-superimposable mirror images are called enantiomers. Enantiomers possess identical physical properties. They only differ with respect to the rotation of plane polarised light. If one of the enantiomer is dextro rotatory, the other will be laevo rotatory. Here, the enantiomer of molecule (A) is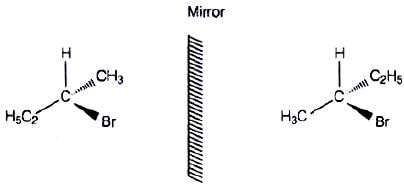 Hence, option (i) is correct.
Hence, option (i) is correct.
Q.11. Which of the following is an example of vic-dihalide?
(i) Dichloromethane
(ii) 1, 2-dichloroethane
(iii) Ethylidene chloride
(iv) Allyl chloride
Ans. (ii)
Solution.
vic-dihalides are those halides in which two halogen atoms are present on the two adjacent carbon atoms.
Structures of the given compounds are as follows: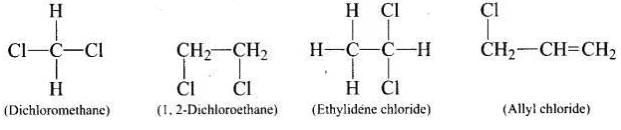 In 1, 2-dichloroethane, the two chlorine atoms are attached to two adjacent carbon atoms.
In 1, 2-dichloroethane, the two chlorine atoms are attached to two adjacent carbon atoms.
Hence, option (ii) is correct.
Q.12. The position of –Br in the compound in CH3CH = CHC(Br)(CH3)2 can be classified as ____________.
(i) Allyl
(ii) Aryl
(iii) Vinyl
(iv) Secondary
Ans. (i)
Solution.
Allyl halides are those compounds in which the halogen atom is bonded to sp3 hybridised carbon atom next to carbon carbon-double bond.
e.g., CH2 = CH —CH2X and CH3CH = CHC(Br) (CH3)2.
Aryl halides are the compounds in which the halogen atom is bonded to the sp2 hybridised carbon atom of an aromatic ring.
e.g.,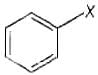 Vinyl halides are the compounds in which the halogen atom is bonded to the sp2 hybridised carbon atom of a carbon double bond.
Vinyl halides are the compounds in which the halogen atom is bonded to the sp2 hybridised carbon atom of a carbon double bond.
e.g.,
CH2 = CH—X
Secondary alkyl halides are the compounds in which the halogen atom is bonded to the sp3 hybridised carbon atom which is further bonded to two alkyl groups and one hydrogen atom
e.g.,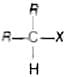
Q.13. Chlorobenzene is formed by reaction of chlorine with benzene in the presence of AlCl3. Which of the following species attacks the benzene ring in this reaction?
(i) Cl–
(ii) Cl+
(iii) AlCl3
(iv) [AlCl4]–
Ans. (ii)
Solution.
In this reaction, AlCl3 is a catalyst which activates the chlorine molecule to show heterolytic cleavage. AlCl3 is electron deficient molecule and forms AlCl−4 and Cl+ when reacts with Cl2. This Cl+ electrophile attacks on electron rich benzene ring.
AlCl3 + Cl2 → [AlCl4]− + Cl+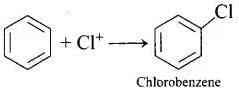
Q.14. Ethylidene chloride is a/an __________.
(i) Vic-dihalide
(ii) Gem-dihalide
(iii) Allylic halide
(iv) Vinylic halide
Ans. (ii)
Solution.
In vic-dihalides, halogen atoms are present on the adjacent carbon atoms.
In gem-dihalides, halogen atoms are present on the same carbon atom. They are known as alkylidene halides
In allylic halides, halogen atom is bonded to sp3 hybridised carbon atom next to carbon-carbon double bond.
In vinylic halides, halogen atom is bonded to sp2 hybridised carbon atom of a carbon-carbon double bond.
In ethylidene chloride (H3C—CHCl2) both halogen atoms are present on same carbon atom so it is gem-dihalide.
Q.15. What is ‘A’ in the following reaction?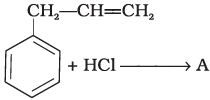
(i)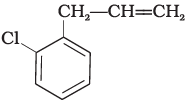
(ii)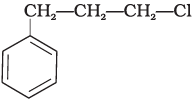
(iii)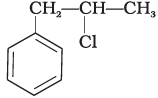
(iv)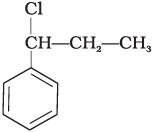
Ans. (iii)
Solution.
In this reaction, addition of HCl takes place on doubly bonded carbons in accordance with Markownikoff's rule i.e., addition of negative addendum will take place on that carbon which has lesser number of hydrogen.
Thus,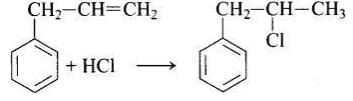 Hence, option (iii) is correct.
Hence, option (iii) is correct.
Q.16. A primary alkyl halide would prefer to undergo ___________.
(i) SN1 reaction
(ii) SN2 reaction
(iii) α–Elimination
(iv) Racemisation
Ans. (ii)
Solution.
A primary alkyl halide would prefer to undergo SN2 reaction.
(a) SN1 reactions occur only if the intermediate carbonation is stable i.e., 3° carbocation.
(b) SN2 reactions occur if there is less steric hinderance on the α-carbon of alkyl halide. In case of primary alkyl halides carbocation is highly unstable and steric hinderance is very less. So, primary alkyl halide would prefer to undergo SN2 reaction.
(c) In α-elimination, proton and the leaving group are present on same atom.
(d) Racemisation is the process of conversion of enantiomer into a racemic mixture.
Q.17. Which of the following alkyl halides will undergo SN1 reaction most readily?
(i) (CH3)3C—F
(ii) (CH3)3C—Cl
(iii) (CH3)3C—Br
(iv) (CH3)3C—I
Ans. (iv)
Solution.
All the given compounds are tertiary alkyl halides but the bond formed between carbon and iodine (C—I) bond is the weakest bond due to large difference in the size of carbon and iodine. So, (CH3)3C—I gives SN1 reaction most readily. In other words, iodine is a better leaving group.
Q.18. Which is the correct IUPAC name for 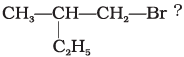
(i) 1-Bromo-2-ethylpropane
(ii) 1-Bromo-2-ethyl-2-methylethane
(iii) 1-Bromo-2-methylbutane
(iv) 2-Methyl-1-bromobutane
Ans. (iii)
Solution.
The correct IUPAC name of the given compound is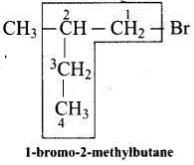
Q.19. What should be the correct IUPAC name for diethyl bromomethane?
(i) 1-Bromo-1,1-diethylmethane
(ii) 3-Bromopentane
(iii) 1-Bromo-1-ethylpropane
(iv) 1-Bromopentane
Ans. (ii)
Solution.
Structure of the diethyl bromomethane is given below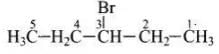 So, the IUPA name is 3-bromopentane.
So, the IUPA name is 3-bromopentane.
Q.20. The reaction of toluene with chlorine in the presence of iron and in the absence of light yields __________.
(i) 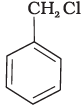
(ii)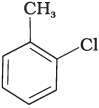
(iii)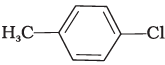
(iv) Mixture of (ii) and (iii)
Ans. (iv)
Solution.
Both ortho – and para – compounds are formed in different yields as depicted below-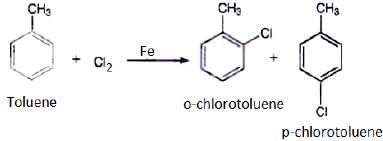
Q.21. Chloromethane on treatment with excess of ammonia yields mainly
(i) N, N-Dimethylmethanamine 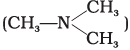
(ii) N–methylmethanamine (CH3—NH—CH3)
(iii) Methanamine (CH3NH2)
(iv) Mixture containing all these in equal proportion
Ans. (iii)
Solution.
Chloromethane on treatment with excess of ammonia yields mainly methamine.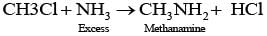
However, if the two reactants are present in the same amount, then the mixture of primary, secondary and tertiary amine is obtained.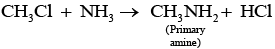
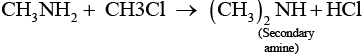
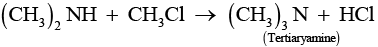
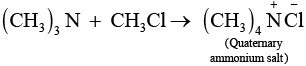
Q.22. Molecules whose mirror image is non superimposable over them are known as chiral. Which of the following molecules is chiral in nature?
(i) 2-Bromobutane
(ii) 1-Bromobutane
(iii) 2-Bromopropane
(iv) 2-Bromopropan-2-ol
Ans. (i)
Solution.
Molecules whose mirror image is non-super imposable over them are known as chiral. It occurs on that molecule which contains asymmetric carbon or chiral (i.e., a carbon atom attached with four different groups). In the present case a molecule of 2- Bromobutane [CH3 CH2 C*H (Br) CH3] is a chiral molecule because,
(i) It contains a chiral carbon atom marked as (C*) .
(ii) Its mirror image is non-superimposable over them.
Q.23. Reaction of C6H5CH2Br with aqueous sodium hydroxide follows _________.
(i) SN1 mechanism
(ii) SN2 mechanism
(iii) Any of the above two depending upon the temperature of reaction
(iv) Saytzeff rule
Ans. (i)
Solution.
SN1 mechanism depends upon the stability of carbocation. Higher the stability of carbocation higher will be the possibility of SN1 mechanism to take place. In the given
Compound C5H5CH2Br, carbocation is C6H5CH2. This carbocation C6H6CH2 is a stable carbocation due to resonance, therefore, its showSN1 mechanism.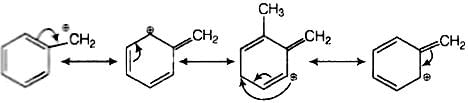
Q.24. Which of the carbon atoms present in the molecule given below are asymmetric?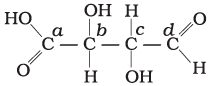
(i) a, b, c, d
(ii) b, c
(iii) a, d
(iv) a, b, c
Ans. (ii)
Solution.
Carbon has four valencies. If a carbon atom satisfies all of its four valencies with four different groups then it is termed asymmetric/chiral carbon. In the given compound, b and c carbon are bonded to four different groups, so these are asymmetric.
Q.25. Which of the following compounds will give racemic mixture on nucleophilic substitution by OH– ion?
(a)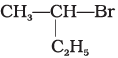
(b)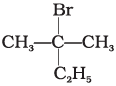
(c)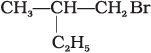
(i) (a)
(ii) (a), (b), (c)
(iii) (b), (c)
(iv) (a), (c)
Ans. (i)
Solution.
A mixture containing two enantiomers in equalimolar amount has zero optical rotion as the rotation due to one isomer is cancelled by the rotation due to other isomar. Such mixture is known as racemic mixture. All those compounds which follow SN1 mechanism during nucleophilic substitution reaction form racemic mixture. Order of reactivity halides towards SN1 reactions is as follows:
Tertiary halide > secondary halides > primary halide
Here compound (a) contains a chiral carbon and gives a racemic product. So, it will give racemic mixture.
Hence, option (i) is correct.
Q.26. In the question arrange the compounds in increasing order of rate of reaction towards nucleophilic substitution.
(a)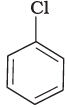
(b)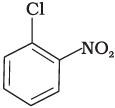
(c)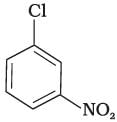
(i) (a) < (b) < (c)
(ii) (c) < (b) < (a)
(iii) (a) < (c) < (b)
(iv) (c) < (a) < (b)
Ans. (iii)
Solution.
The bond formed between C of benzene ring and halogen is more stable because of resonance it has partial double bond character. So, rate of reaction towards nucleophilic substitution is slow. This substitution is facilitated by the presence of electron withdrawing group at ortho and para position because electron density is high at these positions.
Compound (b) and (c) both has one electron withdrawing group but in compound (b) electron withdrawing (—NO2) group is present at ortho position, so rate of reaction in compound (b) is more than that of (c) while (a) has no electron withdrawing group.
Hence, the correct option is (iii).
Q.27. In the question arrange the compounds in increasing order of rate of reaction towards nucleophilic substitution.
(a) 
(b) 
(c)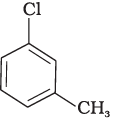
(i) (a) < (b) < (c)
(ii) (a) < (c) < (b)
(iii) (c) < (b) < (a)
(iv) (b) < (c) < (a)
Ans. (iv)
Solution.
Presence of electron releasing group ortho or para position decreases the rate of nucleophilic substitution reaction, in compound (c), electron releasing group is present at meta position w.r.t. chlorine BC re impact is less but in compound (b) it is present at ortho position.
Thus, the rate of reaction towards nucleophilic substation is least in compound (b) and highest in compound (a) as there is no electron releasing group in this compound.
Q.28. In the question arrange the compounds in increasing order of rate of reaction towards nucleophilic substitution.
(a)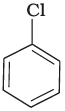
(b)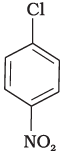
(c) 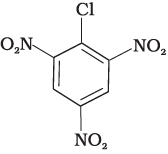
(i) (c) < (b) < (a)
(ii) (b) < (c) < (a)
(iii) (a) < (c) < (b)
(iv) (a) < (b) < (c)
Ans. (iv)
Solution.
Presence of electron withdrawing group at ortho and para position facilitate the nucleophilic substitution reaction and hence, enhances rate of reaction.
Compound (c) has three electron withdrawing groups at ortho and para positions w.r.t. chlorine while compound (b) has only one electron withdrawing group and there is no electron withdrawing group in compound (a). So, the increasing order of rate of reaction towards nucleophilic substitution is (a) < (b) < (c).
Q.29. In the question arrange the compounds in increasing order of rate of reaction towards nucleophilic substitution.
(a)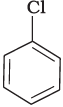
(b)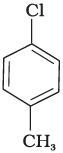
(c)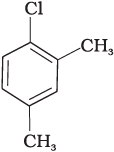
(i) (a) < (b) < (c)
(ii) (b) < (a) < (c)
(iii) (c) < (b) < (a)
(iv) (a) < (c) < (b)
Ans. (iii)
Solution.
Presence of electron releasing group at ortho and para position w.r.t. to chlorine decreases the rate of nucleophilic substitution reaction. Compound (c) has two electron releasing groups and compound (b) has one electron releasing group w.r.t. chlorine while compound (a) has no electron releasing group.
So, the rate of nucleophilic substitution reaction is highest in compound (a) and order is (c) < (b) < (a)
Q.30. Which is the correct increasing order of boiling points of the following compounds?
1-Iodobutane, 1-Bromobutane, 1-Chlorobutane, Butane
(i) Butane < 1-Chlorobutane < 1-Bromobutane < 1-Iodobutane
(ii) 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane < Butane
(iii) Butane < 1-Iodobutane < 1-Bromobutane < 1-Chlorobutane
(iv) Butane < 1-Chlorobutane < 1-Iodobutane < 1-Bromobutane
Ans. (i)
Solution.
Higher the surface area, higher will be the intermolecular forces of attraction and thus boiling point too. Boiling point increases with increase in molecular mass of halogen atom for the similar type of alkyl halide. Butane has no halogen atom and rests of all three compounds are halo derivatives of butane.
Atomic mass of iodine is highest so the boiling point of 1-iodobutane is maximum among all the given compounds and hence, option (i) incorrect.
Q.31. Which is the correct increasing order of boiling points of the following compounds?
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
(i) Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane
(ii) Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane
(iii) 1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene
(iv) 1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Ans. (iv)
Solution.
Boiling point increases with increase in size of hydrocarbon part for the same haloalkanes. All the given haloalkanes contain same halogen atom i.e., bromine but the number of carbon atoms in hydrocarbon part of the molecule are increasing from ethane to benzene.
So the boiling point is minimum for 1-bromoethane and maximum for 1-bromobenzene.
MULTIPLE CHOICE QUESTIONS (TYPE - II)
Note : In the following questions two or more options may be correct.
Consider the following reaction and answer the questions no. 32–34.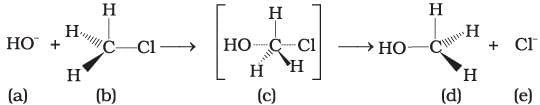
Q.32. Which of the statements are correct about above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridised.
(iii) In (c) carbon atom is sp2 hybridised.
(iv) (a) and (e) both are electrophiles.
Ans. (i, iii)
Solution.
In the above reaction, OH− and Cl− both are electron rich species as they are holding the negative charge. So, they are nucleophiles.
The above reaction shows SN2 mechanism, carbon of alkyl halide is sp3 hybridised.
During this mechanism, the breaking of C—X bond and formation of new bond (C—Nu) occurs simultaneously through a transition state in which carbon atom is approximately sp2 hybridised.
Q.33. Which of the following statements are correct about this reaction?
(i) The given reaction follows SN2 mechanism.
(ii) (b) and (d) have opposite configuration.
(iii) (b) and (d) have same configuration.
(iv) The given reaction follows SN1 mechanism.
Ans. (i, ii)
Solution.
In the given reaction, alkyl halide is primary in nature Here, a transitory state is observed in which one bond is broken and one bond is formed synchronously i.e., in one step. So, it follows SN2 mechanism. In this mechanism, nucleophile attacks the carbon at 180°: to the leaving group, so the reactant and product have opposite configuration
Q.34. Which of the following statements are correct about the reaction intermediate?
(i) Intermediate (c) is unstable because in this carbon is attached to 5 atoms.
(ii) Intermediate (c) is unstable because carbon atom is sp2 hybridised.
(iii) Intermediate (c) is stable because carbon atom is sp2 hybridised.
(iv) Intermediate (c) is less stable than the reactant (b).
Ans. (i, iv)
Solution.
For the given reaction, intermediate (c) represent transition state, and it is highly unstable. In this transition state, carbon atom is sp2 hybridised as partially bonded to two nucleophiles so it is highly unstable and less stable than the reactant (b) Reactant (b).carbon atom is sp3 hybridised and more stable than intermediate (c).
Answer Q. No. 35 and 36 on the basis of the following reaction.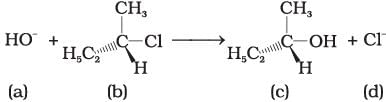 Q.35. Which of the following statements are correct about the mechanism of this reaction?
Q.35. Which of the following statements are correct about the mechanism of this reaction?
(i) A carbocation will be formed as an intermediate in the reaction.
(ii) OH– will attach the substrate (b) from one side and Cl– will leave it simultaneously from other side.
(iii) An unstable intermediate will be formed in which OH– and Cl– will be attached by weak bonds.
(iv) Reaction proceeds through SN1 mechanism.
Ans. (i, iv)
Solution.
The reactant Involve above reaction is secondary alkyl halide. This 2° alkyl halide contain bulky group that’s why it follow SN1 mechanism instead of SN2 mechanism. In SN2 mechanism SN1 mechanism, a stable carbocation will be formed as an intermediate. It is further attacked by OH− nucleophile.
Q.36. Which of the following statements are correct about the kinetics of this reaction?
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Ans. (i, iii)
Solution.
The above reaction follows SN1 mechanism. In SN1 mechanism formation of carbocation is a slow step. Since the rate of reaction depends upon the slowest step, the rate of reaction depends only on the concentration of alkyl halide and not on the concentration of hydroxide ion. So, the rate of reaction depends upon the concentration of (b). Therefore, molecularity of reaction is one.
Q.37. Haloalkanes contain halogen atom (s) attached to the sp3 hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds.
(i) 2-Bromopentane
(ii) Vinyl chloride (chloroethene)
(iii) 2-chloroacetophenone
(iv) Trichloromethane
Ans. (i, iv)
Solution.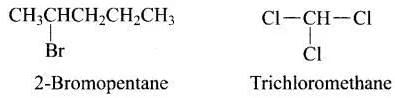 In the structure of 2-bromopentane and trichloromethane, halogen atom is attached to sp3 hybridised carbon atom of an alkyl group. Hence both are haloalkanes. In vinyl chloride and 2-chloroacetophenone, halo group is attached to sp 2 carbon.
In the structure of 2-bromopentane and trichloromethane, halogen atom is attached to sp3 hybridised carbon atom of an alkyl group. Hence both are haloalkanes. In vinyl chloride and 2-chloroacetophenone, halo group is attached to sp 2 carbon.
Q.38. Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form same product on treatment with alcoholic KOH.
(ii) Both the compounds form same product on treatment with aq.NaOH.
(iii) Both the compounds form same product on reduction.
(iv) Both the compounds are optically active.
Ans. (i, iii)
Solution.
(a) Ethylene chloride and ethylidene chloride on treatment with ale. KOH show elimination reaction and ethyne as the product.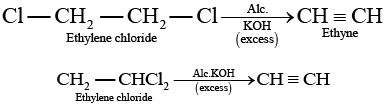 (b) Both these compounds form different products on treatment with aq NaOH.
(b) Both these compounds form different products on treatment with aq NaOH.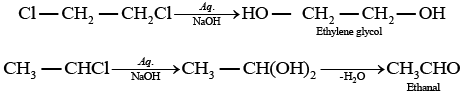 (c) Both these compounds form same products on reduction
(c) Both these compounds form same products on reduction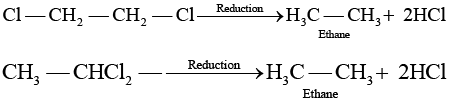 (d) Both these compounds are optically inactive
(d) Both these compounds are optically inactive
Q.39. Which of the following compounds are gem-dihalides?
(i) Ethylidene chloride
(ii) Ethylene dichloride
(iii) Methylene chloride
(iv) Benzyl chloride
Ans. (i, iii)
Solution.
Gem-dihalides are those dihalides in which two halogen atoms are bonded to the same carbon atom.
Write the structure of the given compounds.
(i) 
(ii) 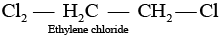
(iii)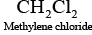
(iv)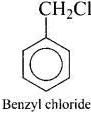
So, in option (i) and (iii) two halogen atoms are present on the same carbon atom and they are termed as gem-dihalides.
Q.40. Which of the following are secondary bromides?
(i) (CH3)2 CHBr
(ii) (CH3)3C CH2Br
(iii) CH3CH(Br)CH2CH3
(iv) (CH3)2CBrCH2CH3
Ans. (i, iii)
Solution.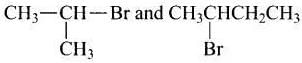 are secondary bromides.
are secondary bromides.
Q.41. Which of the following compounds can be classified as aryl halides?
(i) p-ClC6H4CH2CH(CH3)2
(ii) p-CH3CHCl(C6H4)CH2CH3
(iii) o-BrH2C—C6H4CH(CH3)CH2CH3
(iv) C6H5—Cl
Ans. (i, iv)
Solution.
Aryl halides: These are the compounds in which the halogen atom is bonded to the sp 2 - hybridised carbon atom of an aromatic ring.
Q.42. Alkyl halides are prepared from alcohols by treating with
(i) HCl + ZnCl2
(ii) Red P + Br2
(iii) H2SO4 + KI
(iv) All of these
Ans. (i, ii)
Solution.
(a) Alcohol when treated with HCl + ZnCl2 then alkyl halide is formed.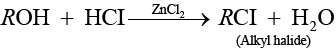
(b) Alcohol when treated with red P and X2 then alkyl halide is formed.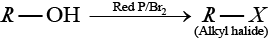
(c) Alcohols when treated with H2SO4 and KI then H2SO4 oxidises KI to I2 and does not produce HI. Therefore, alkyl iodide does not form if the alcohols are treated with H2SO4 + KI.
Q.43. Alkyl fluorides are synthesised by heating an alkyl chloride/bromide in presence of _________ or _________.
(i) Ca F2
(ii) CoF2
(iii) Hg2F2
(iv) NaF
Ans. (ii, iii)
Solution.
Alkyl fluorides are synthesised by alkyl chloride/bromide in presence of CoF2 or Hg2F2. Only transition metal fluorides react with alkyl chloride/bromide to form alkyl fluorides. Alkali metal fluoride such as NaF and alkaline earth metal fluoride such as CaF2 do not react to form fluorides.
SHORT ANSWER TYPE QUESTIONS
Q.44. Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does preparation of aryl iodides requires presence of an oxidising agent?
Ans. lodination reactions are reversible in nature.
C6 H6 + I2 ⇌ C6 H5 I + HI
In this above reaction, hydrogen iodide is formed apart from the required product. It has to be removed from the reaction mixture in order to prevent the backward reaction.
To carry out the reaction in the forward direction, HI formed during the reaction is removed by oxidation using oxidising agent such as HIO3 or HNO3. The reaction is as follows:
5HI + HIO3 → 3I2 + 3H2O;
2HI + 2HNO3 → I + 2NO2 + 2H2O
By using a suitable oxidising agent HI is oxidised to give I2.
Q.45. Out of o-and p-dibromobenzene which one has higher melting point and why?
Ans. p-dibromobenzene has higher melting point than its o-isomer due to symmetry. Due to symmetry, p- isomer fits in the crystal lattice better than the o-isomer.
Hence, p-dibromobenzene has higher melting point.
Q.46. Which of the compounds will react faster in SN1 reaction with the –OH ion?
CH3 — CH2 — Cl or C6H5 — CH2 — Cl
Ans. SN1 mechanism depends upon the stability of carbocation that is formed as intermediate in the mechanism.
CH6 — CH2 — Cl will form C6H5+ CH2 carbocation as intermediate
This carbocation is resonance stabilised and will react faster in SN1 reaction.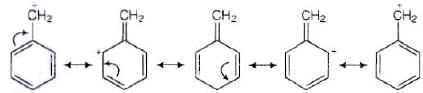 While carbocation formed in CH3CH2Cl is CH3CH2. This carbocation is highly unstable and not give SN1 reaction with −OH ion.
While carbocation formed in CH3CH2Cl is CH3CH2. This carbocation is highly unstable and not give SN1 reaction with −OH ion.
Q.47. Why iodoform has appreciable antiseptic property?
Ans. Iodofrom liberates I2 when it comes in contact with skin. Antiseptic property of iodine is due to the liberation of I2 not because of iodoform itself. (responsible for antiseptic property)
(responsible for antiseptic property)
Q.48. Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
Ans. Due to resonance, C–X bond in haloarenes and haloalkenes have some double bond double bond character. This partial double bond character of C–X bond strengthens the bond. So, haloarenes and haloalkenes are less reactive than haloalkanes.
Let us see the resonating structure of the haloarenes and haloalkenes: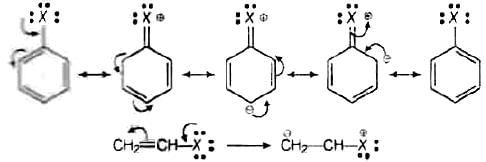 Now, more the number resonating structure higher will be the stability of the compound and lesser will be its reactivity. In haloarenes, more resonating structures are observed than the haloalkenes. So, haloarenes are less reactive than haloarenes.
Now, more the number resonating structure higher will be the stability of the compound and lesser will be its reactivity. In haloarenes, more resonating structures are observed than the haloalkenes. So, haloarenes are less reactive than haloarenes.
Q.49. Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
Ans. Lewis acids are electron deficient species. They are responsible for inducing heterolytic fission in halogen molecules.
Role of Lewis acid is to produce electrophile. The electrophile produced will attack on electron rich benzene to produce aryl bromides and chlorides.
AlCl3 + Cl2 → [AlCl4]−+ Cl+
AlBr3 + Br2 → [AIBr4]− + Br+
This electrophile will further attack on benzene to form respective aryl halide.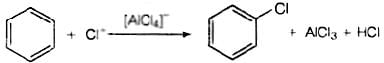
Q.50. Which of the following compounds (a) and (b) will not react with a mixture of NaBr and H2SO4. Explain why?
(a) CH3CH2CH2OH
(b)
Ans. Partial double bond character of a bond increases the strength of the bond and hence, decreases the stability. Phenol will not react with a mixture of NaBr and H2SO4 because it is resonance stabilised. Due to resonance, partial double bond character arises in C–O bond of phenol and it becomes more stable than alcohol.
Reaction 2NaBr + 3H2SO4 → 2NaHSO4 + SO2 + Br2 + 2H2O
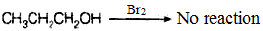 Q.51. Which of the products will be major product in the reaction given below?Explain
Q.51. Which of the products will be major product in the reaction given below?Explain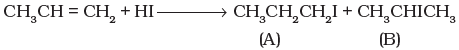 Ans. In the given reaction, (B) is the major product of the reactions. According to Markownikoff’s rule, H is added to the C atom with higher number of hydrogen atoms.
Ans. In the given reaction, (B) is the major product of the reactions. According to Markownikoff’s rule, H is added to the C atom with higher number of hydrogen atoms.
Q.52. Why is the solubility of haloalkanes in water very low?
Ans. Haloalkanes are slightly soluble in water. For the solubility to haloalkane in water, energy is required to overcome the attractions between its own molecules and to break the bonds between water molecules.
Less energy is released when new attractions are set up between the haloalkanes and the water molecules as these are not as strong as the original hydrogen bonds in water. As a result, the solubility of haloalkanes in water is low.
Q.53. Draw other resonance structures related to the following structure and find out whether the functional group present in the molecule is ortho, para directing or meta directing.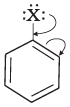 Ans. Resonance in halobenzene is as follows:
Ans. Resonance in halobenzene is as follows: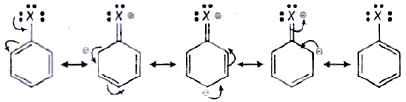 From the above resonating structure it is very clear that electron density is rich at ortho and para position. Therefore, it is ortho and para directing not meta directing.
From the above resonating structure it is very clear that electron density is rich at ortho and para position. Therefore, it is ortho and para directing not meta directing.
Q.54. Classify the following compounds as primary, secondary and tertiary halides.
(i) 1-Bromobut-2-ene
(ii) 4-Bromopent-2-ene
(iii) 2-Bromo-2-methylpropane
Ans. The structural formula of the given compounds are:
(i) 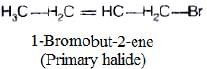
(ii) 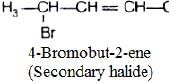
(iii) 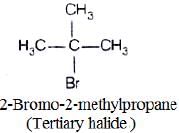
In compound (i), carbon atom to which halogen is bonded, is further bonded to two hydrogen and one carbon of hydrocarbon chain. So, it is primary halide,
In compound (ii), α-carbon is bonded with one hydrogen and two carbons of two hydrocarbon chain. So, it is secondary halide.
In compound (iii) α-carbon is bonded to three alkyl group, so it is tertiary halide.
Q.55. Compound ‘A’ with molecular formula C4H9Br is treated with aq. KOH solution.
The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. KOH solution, the rate of reaction was found to be dependent on concentration of compound and KOH both.
(i) Write down the structural formula of both compounds ‘A’ and ‘B’.
(ii) Out of these two compounds, which one will be converted to the product with inverted configuration.
Ans. (i) As the rate of reaction depends upon the concentration of compound 'A' (C4H9Br) only therefore, the reaction is proceeded by SN1 mechanism and the given compound will be tertiary alkyl halide i.e., 2-bromo -2 methylpropane and the structure is as follow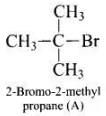 Optically active isomer of (A) is 2-bromobutane (B) and its structural formula is
Optically active isomer of (A) is 2-bromobutane (B) and its structural formula is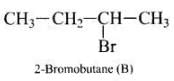 (ii) As compound (B) is optically active therefore, compound (B) must be 2-bromobutane.
(ii) As compound (B) is optically active therefore, compound (B) must be 2-bromobutane.
Since, the rate of reaction of compound (B) depends both upon the concentration of compound (B) and KOH, hence, the reaction follow SN2 mechanism. In SN2 reaction, nucleophile attack from, the back side, therefore, the product of hydrolysis will have opposite configuration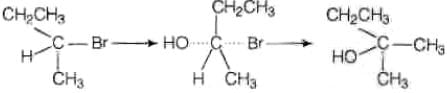
Q.56. Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, C7H8 is treated with Cl2 in the presence of FeCl3.
Ans. When compound ‘A’ with molecular formula C7H8 is treated with Cl2 in the presence of FeCl3 o-chlorotoluene or p-chlorotoluene will be formed as the compound A with molecular formula C7H8 is toluene.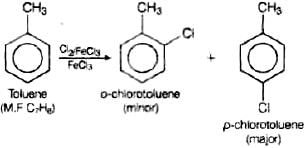
Q.57. Identify the products A and B formed in the following reaction :
(a) CH3—CH2—CH= CH—CH3+HCl → A + B
Ans. In the given reaction, addition occur and the following two products (A and B) are possible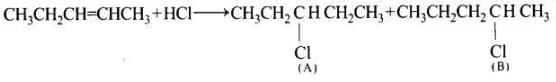
Further the carbocation formed from compound (A) is slightly less stable than carbocation leading to the formation of compound (B) therefore the amount of 2-chloropentane (S) will be slightly more than that of 3-chloropentane (A).
Q.58. Which of the following compounds will have the highest melting point and why?
(I)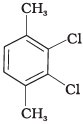
(II)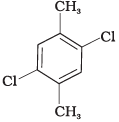
(III)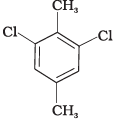
Ans. In compound (II), both the CH3 groups and Cl atoms at para-position to each other. Therefore, compound (II) is more symmetrical and it fits in the crystal lattice better than the other two isomers and hence it has the highest melting point than the others.
Q.59. Write down the structure and IUPAC name for neo-pentylbromide.
Ans. The structure of neo-pentylbromide is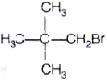 IUPAC name ⇒ l-bromo -2, 2-dimethylpropane
IUPAC name ⇒ l-bromo -2, 2-dimethylpropane
Common name ⇒ neo-pentylbromide
Q.60. A hydrocarbon of molecular mass 72 g mol–1 gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.
Ans. Since, the molar mass of hydrocarbon is 72 g mol−1 thats why the hydrocarbon is C5H12 i.e., pentane.
On photo chlorination, it gives monochloro derivatives so, all the hydrogen atoms must be equivalent and the structure of the compound will be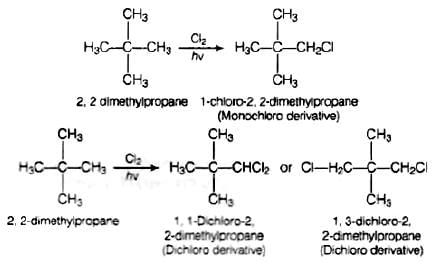
Q.61. Name the alkene which will yield 1-chloro-1-methylcyclohexane by its reaction with HCl. Write the reactions involved.
Ans. Two alkenes are possible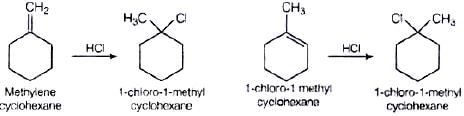 Addition takes place in accordance with Markownikoff's rule i.e., negative part of the adding molecule will get attached to that carbon which has lesser number of hydrogen atom.
Addition takes place in accordance with Markownikoff's rule i.e., negative part of the adding molecule will get attached to that carbon which has lesser number of hydrogen atom.
Q.62. Which of the following haloalkanes reacts with aqueous KOH most easily?
Explain giving reason.
(i) 1-Bromobutane
(ii) 2-Bromobutane
(iii) 2-Bromo-2-methylpropane
(iv) 2-Chlorobutane
Ans. 2-Bromo-2-methylpropane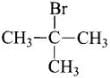 will react the aqueous KOH most easily because the corbocation formed during hte reaction is tertiary and most stable.
will react the aqueous KOH most easily because the corbocation formed during hte reaction is tertiary and most stable.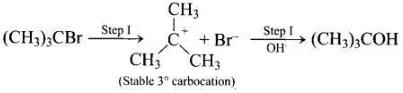
Q.63. Why can aryl halides not be prepared by reaction of phenol with HCl in the presence of ZnCl2?
Ans. Due to resonance in phenol, C—O bond of phenol has some partial double bond character Partial double bond character strengthen the bond. So, it is difficult to break this C — O bond of phenol while the C — O bond of alcohol is purely single bond and comparatively weaker bond.
So alkyl halides can be prepared by the reaction of alcohols with HCl in the presence of ZnCl2 while aryl halides can not be prepared by reaction of phenol with HCl in the presence of ZnCI2.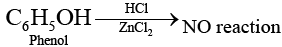
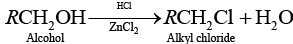
Q.64. Which of the following compounds would undergo SN1 reaction faster and why?
(A) 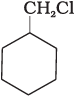
(B)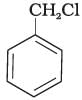
Ans. Compound (B) will give SN1 reaction faster than compound (A) because SN1 reaction depends upon the stability of carbacation. Benzyl chloride on ionisation gives C6H5CH2 carbocation which is resonance srabilised while the carbocation obtained from compound (A) is not stabilised by resonance.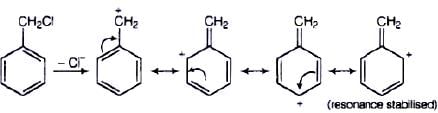
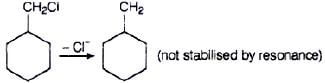
Q.65. Allyl chloride is hydrolysed more readily than n-propyl chloride. Why?
Ans. As we know that SN1 mechanism depends upon the stability of carbocation, Allyl chloride on hydrolysis gives resonance stabilised carbocation while no resonance is observed in the carbocation formed by n-propyl chloride.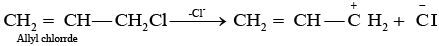
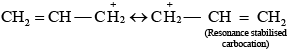
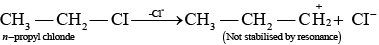
Hence, allyl chloride undergoes hydrolysis much faster than n-propyl chloride.
Q.66. Why is it necessary to avoid even traces of moisture during the use of a Grignard reagent?
Ans. Grignard reagents are highly reactive and react with water to give corresponding hydrocarbons.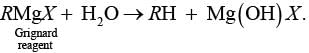
Q.67. How do polar solvents help in the first step in SN1 mechanism?
Ans. Polar solvents help in the first step in SN1 mechanism because leaving group and caroocation both are stabilised by polar solvent, Polarity of a solvent depends upon the value of dielectric constant Higher the value of dielectric constant, higher will be the polarity of the solvent, raster will be the rate of SN1 be the rate of SN1 mechanism. These polar solvents can work as a nucleophile and stabilize the carbocation as follows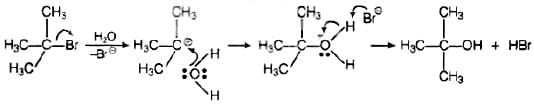
Q.68. Write a test to detect the presence of double bond in a molecule.
Ans. Presence of double bond in a molecule is detected by following two methods:
(i) Br2 in CCl4 test When Br2 /CC|4 is added unsaturated compound then orange colour of bromine disappears and dibromoderivative is formed, (colourless).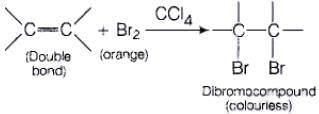 (ii) Bayer's test When alkaline solution of KMnO4 is added to the solution of unsaturated compound then its pink colour disappears due to the formation of dihydroxy derivative.
(ii) Bayer's test When alkaline solution of KMnO4 is added to the solution of unsaturated compound then its pink colour disappears due to the formation of dihydroxy derivative.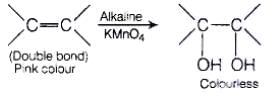
Q.69. Diphenyls are potential threat to the environment. How are these produced from arylhalides?
Ans. In environment, diphenyl is formed during the incomplete combustion of mineral oil and coal. It is present in the exhaust gases of vehicles and in exhaust air from residential and industrial heating devices,
Acute exposure to high levels of biphenyl has been observed to cause eye and skin irritation and toxic effects on the liver, kidneys and central /peripheral nervous system. Kidneys of animals are also affected due to the ingestion of biphenyls. In rats, fetofoxicity has been oserved if they are exposed to high levels of biphenyl.
Preparation of diphenyls from aryl halides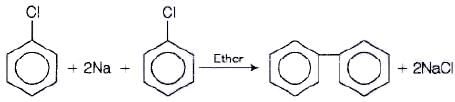
Aryl halides, when treated with sodium in dry ether give diphenyl. This reaction is named as Fittig reaction.
Q.70. What are the IUPAC names of the insecticide DDT and benzene hexachloride? Why is their use banned in India and other countries?
Ans. The IUPAC name of DDT is 2,2-bis (4-chlorophenyl)-1,1,1-trichloroethane and that of benzene hexachloride is 1,2,3,4,5,6-hexachlorocyclohexane.
They are banned in India because they are non-biodegradable. Instead, they get deposited and stored in fatty tissues. If this ingestion continues at a steady rate, DDT builds up within the animal over time. This will affect the reproductive system of animals.
If animals including humans are exposed to high levels of benzene hexachloride then it may cause acute poisoning. Apart from that this BHC may affect liver functioning in humans.
Q.71. Elimination reactions (especially β-elimination) are as common as the nucleophilic substitution reaction in case of alkyl halides. Specify the reagents used in both cases.
Ans. Elimination reactions are as common as the nucleophilic substitution reaction in case of alkyl halides as two reactions occur simultaneously. Generally, at lower temperature and by using weaker base, nucleophilic substitution reaction occurs while at higher temperature and by using a stronger base, elimination reactions (especially b-elimination) take place. e.g., if ethyl bromide is treated with aq. KOH, at low temperature it gives ethanol while if it is treated with alc. KOH at high temperature then it gives ethene.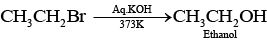 (Nucleophilic substitution reaction)
(Nucleophilic substitution reaction)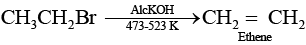 (Elimination reaction)
(Elimination reaction)
Q.72. How will you obtain monobromobenzene from aniline?
Ans. When aniline, dissolved or suspended in cold aqueous mineral acid, is treated with sodium nitrite, a diazonium salt is formed. This diazo salt on treatment with cuprous bromide gives monobromobenzene.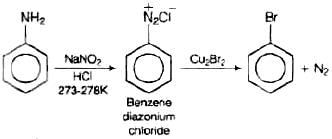 This reaction is named as Sandmeyer's reaction. If benzene diazonium chloride is treated with copper in HBr then the product formed is bromobenzene. This reaction is known as Gattermann reaction.
This reaction is named as Sandmeyer's reaction. If benzene diazonium chloride is treated with copper in HBr then the product formed is bromobenzene. This reaction is known as Gattermann reaction.
Q.73. Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution:
(I) 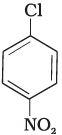
(II)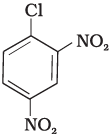
(III)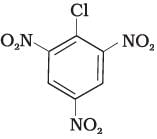
Ans. Aryl halides are less reactive towards nucleophilic substitution reaction. Presence of electron withdrawing group at ortho and para position increases the stability of intermediates and hence increases the reactivity of aryl halides towards nucleophilic substitution reaction.
Now, more the number of electron withdrawing groups (EWG) at ortho and para position, higher will be the reactivity of aryl halide. Compound (III) has three EWG so, it is most reactive and compound (I) has only one EWG so, it is least reactive, So, the order of reactivity is (I) < (II) < (III)
Q.74. tert-Butylbromide reacts with aq. NaOH by SN1 mechanism while n-butylbromide reacts by SN2 mechanism. Why?
Ans. Tert. butyl bromide reacts with aq. NaOH as follows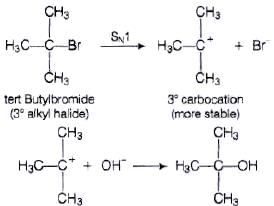
tert. butyl bromide when treated with aq. NaOH, it forms tert. corbocation which is more stable intermediate. This intermediate is further attacked by −OH ion. As tert. carbocation is highly stable so tert butylbromide follow SN1 mechanism.
In case of n-butylbromide, primary carbocation is formed which is least stable so, it does not follow SN1 mechanism. Here, stearic hindrance is very less so, it follows SN2 mechanism. In SN2 mechanism, −OH will attack from backside and a transition state is formed. The leaving group is then pushed off the opposite side and the product is formed.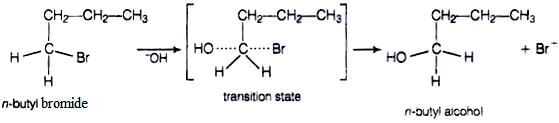
Q.75. Predict the major product formed when HCl is added to isobutylene. Explain the mechanism involved.
Ans. Reaction between the isobutylene added to HCl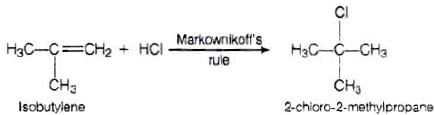
Electrophilic addition reaction takes place in accordance with Markownikoff s rule,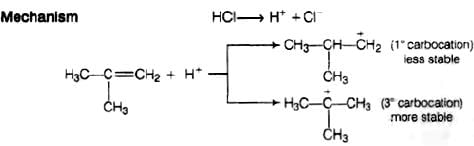
We know that 3° carbocation is more stable than 1° carbocation, so in further step 3° carbocation is further attacked by Cl− ion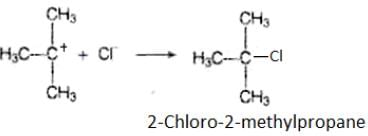
Q.76. Discuss the nature of C–X bond in the haloarenes.
Ans. In haloarenes, carbon of benzene is bonded to halogen. Electronegativity of halogen is more than that of sp2 hybridised carbon of benzene ring. So, C–X bond is a polar bond. Apart from this, lone pair of electrons of halogen atom is involved in resonance with benzene ring. So, this C–X bond has acquired partial bond character.
This C–X bond of haloarenes is less polar than C–X bond of haloalkanes. This is supported by the fact that dipole moment of chlorobenzene (μ = 1.69D) is little lower than that of CH3Cl (μ = 1.83D).
Q.77. How can you obtain iodoethane from ethanol when no other iodine containing reagent except NaI is available in the laboratory?
Ans. Ethanol is treated with red phosphorous and bromine mixture and the product formed will be bromoethane. Bromoethane so formed is then treated with NaI to give lodoethane.
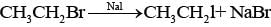
Thus reaction is known as Finkelstein reaction
Q.78. Cyanide ion acts as an ambident nucleophile. From which end it acts as a stronger nucleophile in aqueous medium? Give reason for your answer.
Ans. Cyanide ion (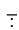 C ≡ N) is an ambident nucleophile because it can react either through carbon or through nitrogen. Since, C — C bond is stronger than C — N bond so, cyanide ion will mainly attack through carbon to form alkyl cyanide.
C ≡ N) is an ambident nucleophile because it can react either through carbon or through nitrogen. Since, C — C bond is stronger than C — N bond so, cyanide ion will mainly attack through carbon to form alkyl cyanide.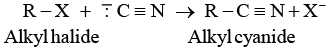
MATCHING TYPE
Note : Match the items given in Column I and Column II in the following questions.
Q.79. Match the the compounds given in Column I with the effects given in Column II.
| Column I | Column II |
| (i) Chloramphenicol | (a) Malaria |
| (ii) Thyroxine | (b) Anaesthetic |
| (iii) Chloroquine | (c) Typhoid fever |
| (iv) Chloroform | (d) Goiter |
| (e) Blood substituent |
Ans. i → (c), ii → (d), iii → (a), iv → (b)
Solution.
(i) Chloramphenicol is a broadspectrum antibiotic. It is used in the treatment of typhoid fever.
(ii) Thyroxine is a hormone secreated by thyroid gland. Execessive secretion of thyroxine in the body is known as hyperthyroidism. Most patient with hyper thyroidism have an enlarged thyroid gland i.e., goitre.
(iii) Chloroquine prevents the development of malaria parasite Plasmodium vivax in the blood.
(iv) IUPAC name of chloroform is trichloromethane with formula CHCl3. It is a colourless, volatile, sweet-smelling liquid. Its vapours depresses the central nervous system and used as an anaesthetic.
Q.80. Match the items of Column I and Column II.
| Column I | Column II |
| (i) SN1 reaction | (a) vic-dibromides |
| (ii) Chemicals in fire extinguisher | (b) gem-dihalides |
| (iii) Bromination of alkenes | (c) Racemisation |
| (iv) Alkylidene halides | (d) Saytzeff rule |
| (v) Elimination of HX from alkylhalide | (e) Chlorobromocarbons |
Ans. i → (c), ii → (e), iii → (a), iv → (b), v → (d)
Solution.
(i) A mixture containing two enantiomers in equal proportions will have zero optical rotation, such a mixture is known as racemic mixture. The process of conversion of enantiomer into a racemic mixture is known as racemisation. If an alkyl halide follows
SN1 mechanism then racemisation takes place while if it follows SN2 mechanism than inversion takes places.
(ii) Chlorobromocarbons are used in fire extinguishers,
(iii). In vicinal dihalides, halogen atoms are present on the adjacent carbon atom. Bromination of alkenes will give vicinal dihalides.
(iv) Alkylidene haiides are named as gem-dihalides. In gem-dihalides halogen atoms are present on same carbon atom.
(v) Elimination of HX from alkylhalide follows Saytzeff rule. This rule states that "in dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms".
Q.81. Match the structures of compounds given in Column I with the classes of compounds given in Column II.
| Column I | Column II |
(i) 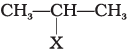 | (a) Aryl halide |
| (ii) CH2 = CH—CH2—X | (b) Alkyl halide |
(iii) 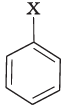 | (c) Vinyl halide |
| (iv) CH2 = CH—X | (d) Allyl halide |
Ans. i → (b), ii → (d), iii → (a), iv → (c)
Solution.
(i) In alkyl halide, halogen atom is bonded to sp3 hybridised carbon atom, which may be further bonded to one, two or three alkyl groups, i.e., CH3−CH(X) −CH3
(ii) Allyl halides are the compounds in which the halogen atom is bonded to sp3 hybridised carbon atom next to carbon-carbon double bond, i.e., CH2− =CH −CH2−X
(iii) Aryl halides are the compounds in which the halogen atom is bonded to sp2 hybridised carbon atom of an aromatic ring, i.e., C6H5X
(iv) Vinyl halides are the compounds in which the halogen atom is bonded to an sp2 hybridised carbon atom of a carbon-carbon double bond, i.e., CH2 =CH−X
Q.82. Match the reactions given in Column I with the types of reactions given in Column II.
| Column I | Column II |
(i) 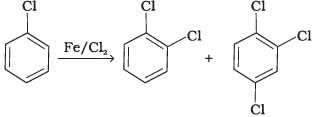 | (a) Nucleophilic aromatic substitution |
(ii) 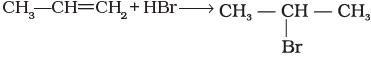 | (b) Electrophilic aromatic substitution |
(iii) 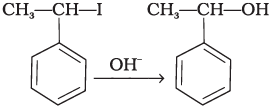 | (c) Saytzeff elimination |
(iv) 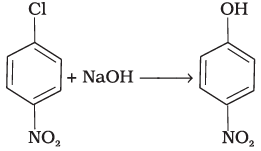 | (d) Electrophilic addition |
(v) 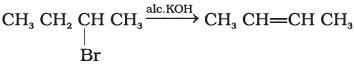 | (e) Nucleophilic substitution (SN1) |
Ans. i → (b), ii → (d), iii → (e), iv → (a), v → (c)
Solution.
(i) In this reaction, an electrophile attacks on the benzene ring and substitution takes place.
(ii) In this reaction, addition of HBr takes place on to the doubly bonded carbons of propene in accordance with Markownikoff's rule and electrophilic addition takes place.
(iii) In this reaction, the reactant is secondary halide. Here, halogen atom is substituted by hydroxy ion. As it is secondary halide so it follows SN1 mechanism.
(iv) In this reaction, halogen atom is directly bonded to aromatic ring. So, It is nucieophilic aromatic substitution as –OH group has substituted halogen of given compound.
(v) It is an elimination reaction. It follows Saytzeff elimination rule.
Q.83. Match the structures given in Column I with the names in Column II.
| Column I | Column II |
(i) 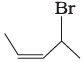 | (a) 4-Bromopent-2-ene |
(ii) 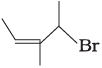 | (b) 4-Bromo-3-methylpent-2-ene |
(iii) 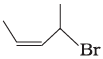 | (c) 1-Bromo-2-methylbut-2-ene |
(iv) 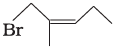 | (d) 1-Bromo-2-methylpent-2-ene |
Ans. i → (a), ii → (b), iii → (c), iv → (d)
Solution.
(i) The IUPAC name of compound (A) is 4-bromopent-2-ene.
(ii) The IUPAC name of compound (B) is 4-bromo-3-methyl pent-2-ene.
(iii) The IUPAC name of compound (C) is 1-bromo-2- methyl but-2-ene.
(iv) The IUPAC name of compound (D) is 1-bromo-2-methylpent-2-ene.
Q.84. Match the reactions given in Column I with the names given in Column II.
| Column I | Column II |
(i) 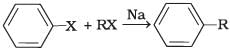 | (a) Fittig reaction |
(ii) 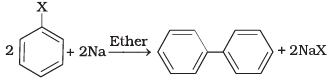 | (b) Wurtz Fittig reaction |
(iii)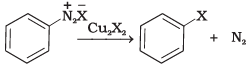 | (c) Finkelstein reaction |
(iv) 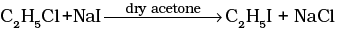 | (d) Sandmeyer reaction |
Ans. i → (b), ii → (a), iii → (d), iv → (c)
Solution.
(i) A mixture of an alkyl hailde and aryl halide gives an alkylarene when treated with sodium in dry ether and this is called Wurtz-Fittig reaction
(ii) Aryl halides give analogous compounds when treated with sodium in dry ether, in which two aryl groups are joined together. It is called Fittig reaction.
(iii) Diazonium salt when treated with cuprous chloride or cuprous bromide gives chlorobenzene or bromobenzene.
The reaction is known as Sandmeyer's reaction
(iv) Alkyl iodides are prepared by the reaction of alkyl chlorides with sodium iodide in dry acetone. The reaction is known as Finkelstein reaction.
ASSERTION AND REASON TYPE
Note : In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Q.85. Assertion: Phosphorus chlorides (tri and penta) are preferred over thionyl chloride for the preparation of alkyl chlorides from alcohols.
Reason: Phosphorus chlorides give pure alkyl halides.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (ii)
Solution.
Correct Assertion Thionyl chloride is preferred over PCl3 and PCl5 for the preparation of alkyl chlorides from alcohols.
Correct Reason Thionyl chloride gives pure alkyl halide as other two products (SO2 + HCl) are escapable gases.
Q.86. Assertion: The boiling points of alkyl halides decrease in the order : RI > RBr > RCl > RF
Reason: The boiling points of alkyl chlorides, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (v)
Solution.
For the same hydrocarbon part boiling point depends upon the atomic mass of halogen atom. Higher the mass of the halogen atom, higher will be the boiling point.
So, we can say that boiling point decreases with decrease in atomic mass of halogen atom.
Q.87. Assertion: KCN reacts with methyl chloride to give methyl isocyanide
Reason: CN – is an ambident nucleophile.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (iv)
Solution.
Correct Assertion KCN reacts with methyl chloride to give the mixture of methyl cyanide and methyl isocyanide in which methyl cyanide predominates because of stable C—C bond in methyl cyanide.
Q.88. Assertion: tert-Butyl bromide undergoes Wurtz r eaction to give 2, 2, 3, 3-tetramethylbutane.
Reason: In Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (iv)
Solution.
Correct Assertion- sec-butyl bromide (2° alkyl halide) undergoes Wurtz reaction to give 2, 5-dimethylhexane.
Q.89. Assertion: Presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution.
Reason: Nitro group, being an electron withdrawing group decreases the electron density over the benzene ring.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (iv)
Solution.
Presence of nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution because —NO2 group, being an electron withdrawing group decreases the electron density over the benzene ring.
Q.90. Assertion: In monohaloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason : Halogen atom is a ring deactivator.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (v)
Solution.
Correct explanation in monohaloarenes, halogen atom increases the electron density at ortho and para position. So, further electrophilic substitution occurs at ortho and para positions.
Q.91. Assertion: Aryl iodides can be prepared by reaction of arenes with iodine in the presence of an oxidising agent.
Reason: Oxidising agent oxidises I2 into HI.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (iii)
Solution.
Correct Reason Oxidising agent oxidises HI into l2 to prevent the possibility of backward reaction.
Q.92. Assertion: It is difficult to replace chlorine by –OH in chlorobenzene in comparison to that in chloroethane.
Reason: Chlorine-carbon (C—Cl) bond in chlorobenzene has a partial double bond character due to resonance.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (i)
Solution.
It is difficult to replace chlorine by —OH in chlorobenzene in comparison to that in chloromethane because C—Cl bond in chlorobenzene has a partial double bond character due to resonance.
Q.93. Assertion: Hydrolysis of (–)-2-bromooctane proceeds with inversion of configuration.
Reason: This reaction proceeds through the formation of a carbocation.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (iii)
Solution.
Correct Reason This reaction proceeds through SN2 mechanism, in which −OH ion attacks at 180° to the halogen atom of 2-bromooctane which leads to the inversion of configuration.
Q.94. Assertion: Nitration of chlorobenzene leads to the formation of m-nitrochlorobenzene
Reason: —NO2 group is a m -directing group.
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
(ii) Assertion and reason both are wrong statements.
(iii) Assertion is correct but reason is wrong statement.
(iv) Assertion is wrong but reason is correct statement.
(v) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
Ans. (iv)
Solution.
Correct Assertion Chlorination of nitrobenzene leads to the formation of mnitrochlorobenzene because —NO2 group deactivates the ring as it is meta directing.
LONG ANSWER TYPE QUESTIONS
Q.95. Some alkylhalides undergo substitution whereas some undergo elimination reaction on treatment with bases. Discuss the structural features of alkyl halides with the help of examples which are responsible for this difference.
Ans. Primary alkyl halides follow SN2 mechanism in which a nucleophile attacks at 180° to the halogen atom. A transition state is formed in which carbon is bonded to two nucleophiles and finally halogen atom is pushed out. In SN2 mechanism, substitution of nucleophile takes place as follows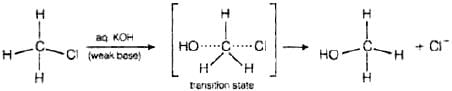 Thus, in SN2 mechanism, substitution takes place. Tertiary alkyl halides follow SN1 mechanism. In this case, tert alkyl halides form 3° carbocations. Now, if the reagent used is a weak base then substitution occurs while if it is a strong base than instead of substitution elimination occurs.
Thus, in SN2 mechanism, substitution takes place. Tertiary alkyl halides follow SN1 mechanism. In this case, tert alkyl halides form 3° carbocations. Now, if the reagent used is a weak base then substitution occurs while if it is a strong base than instead of substitution elimination occurs.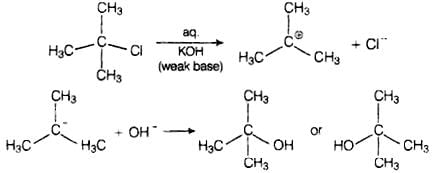 Here, the reagent used is aq. KOH. It is a weak base so, substitution takes place.
Here, the reagent used is aq. KOH. It is a weak base so, substitution takes place.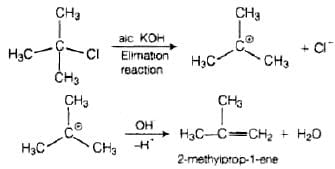 As alc. KOH is a strong base, so elimination competes over substitution and alkene is formed.
As alc. KOH is a strong base, so elimination competes over substitution and alkene is formed.
Q.96. Some halogen containing compounds are useful in daily life. Some compounds of this class are responsible for exposure of flora and fauna to more and more of UV light which causes destruction to a great extent. Name the class of these halocompounds. In your opinion, what should be done to minimise harmful effects of these compounds.
Ans. Some halogen containing compounds are useful in daily life are as follows
Dichloromethane: It is used as a solvent, as a paint remover, as a propellant in aerosols, and as a process solvent in the manufacture of drugs. It is also used as a metal cleaning and finishing solvent.
Trichloromethane: It is employed as a solvent for fats, alkaloids, iodine and other substances.
Triodomethane: It is used as an antiseptic. Now, it has been replaced by some other compounds because of its objectionable smell.
But some compounds of this class are responsible for exposure of flora and fauna to more and more of UV light which causes destruction to great extent.
These are as follows.
(i) Tetrachloromethane: When carbon tetrachloride is released into the air, it rises to the atmosphere and depletes the ozone layer. Depletion of the ozone layer is believed to increase human exposure to UV rays leading to increased skin cancer, eye diseases and disorders, and possible disruption of the immune system. These UV rays cause damage to plants, and reduction of plankton populations in the ocean's photic zone.
(ii) Freons: Freon-113 is likely to remain in the air long enough to reach the upper atomsphere. Here, it provides chlorine atoms which damage the ozone layer. Because of this depletion UV rays enters in our atmosphere and become responsible for damage to great extent.
(iii) p-p'-Dichlorodiphenyl trichloroethane (DDT)
DDT is not completely biodegradable. Instead, it gets deposited in fatty tissues. If ingestion continues for a long time, DDT builds up within the animal and effect the reproductive system.
To minimise the harmful impacts of these compounds i.e., freons, hydrofluorocarbons, fluorocarbons and hydrocarbons can be straight used to make refrigerants and air-conditioning equipments. They are stable in the stratosphere and secure for flora and fauna.
Q.97. Why are aryl halides less reactive towards nucleophilic substitution reactions than alkyl halides? How can we enhance the reactivity of aryl halides?
Ans. Aryl halides are less reactive towards nucleophilic substitution reaction due to the following reasons (i) In haloarenes, the lone pair of electron on halogen are in resonance with benzene ring So, C—Cl bond acquires partial double bond character which strengthens C— Cl bond.
Therefore, they are less reactive towords nucleophilic substitution reaction.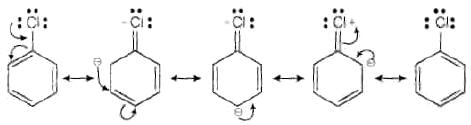
(ii) In haloarenes, the carbon atom attached to halogen is sp2 hybridised. The sp2 hybridised carbon is more electronegative than sp3 hybridised carbon. This sp2 hybridised carbon in haloarenes can hold the electron pair of C—X bond more tightly and make this C — Cl bond shorter than C — Cl bond of haloalkanes.
Since, it is difficult to break a shorter bond than a longer bond, therefore, haloarenes are less reactive than haloarenes.
In haloarenes, the phenyl cation will not be stabilised by resonance therefore SN1 mechanism ruled out.
(iv) Because of the repulsion between the nucleophile and electron rich arenes, aryl halides are less reactive than alkyl halides.
The reactivity of aryl halides can be increased by the presence of an electron withdrawing group (-(NO2) at ortho and para positions. However, no effect on reactivity of haloarenes is observed by the presence of electron withdrawing group at meta-position. Mechanism of the reaction is as depicted with –OH ion.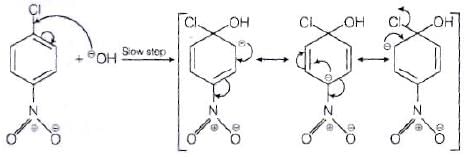
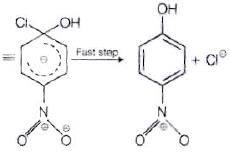
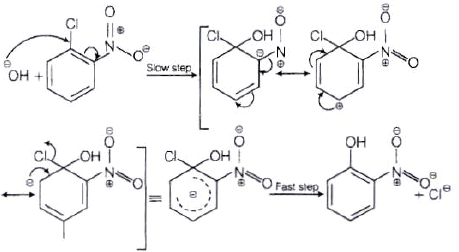
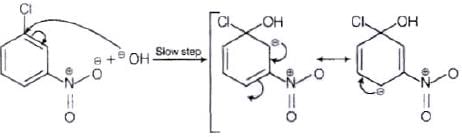
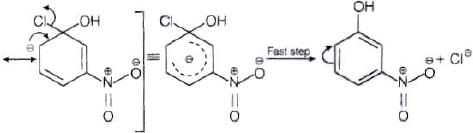 From the above resonance, it is very clear that electron density is rich at ortho and para positions. So, presence of EWG will facilitate nucleophilic at ortho and para postitions not on meta position.
From the above resonance, it is very clear that electron density is rich at ortho and para positions. So, presence of EWG will facilitate nucleophilic at ortho and para postitions not on meta position.
|
352 videos|596 docs|309 tests
|
FAQs on NCERT Exemplar: Haloalkanes and Haloarenes - Chemistry for JEE Main & Advanced
| 1. What are haloalkanes and haloarenes? |  |
| 2. How are haloalkanes and haloarenes named? |  |
| 3. What are the physical properties of haloalkanes and haloarenes? |  |
| 4. How do haloalkanes and haloarenes react? |  |
| 5. What are the uses of haloalkanes and haloarenes? |  |
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|

















