NCERT Solutions for Class 5 EVS Chapter 7 - Experiments with Water
| Table of contents |

|
| Think what would happen if |

|
| Do this and Find out |

|
| Is It Magic? |

|
| What Dissolved and what did not? |

|
| Do this Experiment |

|
| Tell |

|
| Racing Drops |

|
| Where Did the water go? |

|
| What we have Learned |

|
Think what would happen if
Q.1. Ayesha put a puffed puri in a bowl of water. Would it sink or float?Ans.
- If Ayesha puts a puffed puri in a bowl of water, it will float on the surface.
- This is because the puffed puri is light and has air trapped inside it, making it less dense than water.
- Since it is less dense, it will not sink; instead, it will remain on top of the water.
 Soap Bar sinks in Water
Soap Bar sinks in Water
Q.2. You put a steel plate on water. Would it sink or float? What would happen to a spoon?
Ans.
- A steel plate will sink when placed on water.
- Although steel is heavy, the plate’s shape plays an important role. If it is flat and has a large surface area, it may float due to the shape distributing its weight. However, generally, a solid steel plate is likely to sink because its overall density is greater than that of water.
- On the other hand, a spoon made of steel will typically sink in water too because it is also denser than water. Both the steel plate and spoon will go down to the bottom of the bowl.
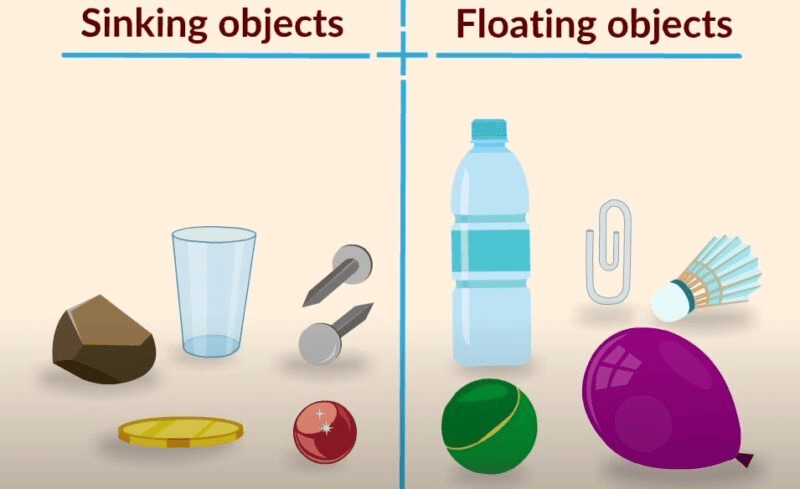 Some Sinking & Floating Objects
Some Sinking & Floating Objects
Q.3. Would the cap of plastic bottle cap sink or float on water?
Ans.
- The cap of a plastic bottle will float on water.
- This is because plastic is less dense than water.
- When placed in water, the cap will not sink; instead, it will stay on the surface, bobbing gently.
Q.4. Have you seen something float on the water while others sinks? Think how this happens!
Ans.
- It is observed that few things float and few sink in the water.
- If the item put in the water is lighter then it floats.
- If it's heavier then it sinks.
- It depends upon the volume of the item.
Do this and Find out
Do this experiment in groups of four friends. Each group will need a big pot filled with water and the things listed on the table. Put each thing one by one in water and observe. Write your observations in the table given below:Q1. Mark [✓] for the things that float. Mark [✕] for those that sink.
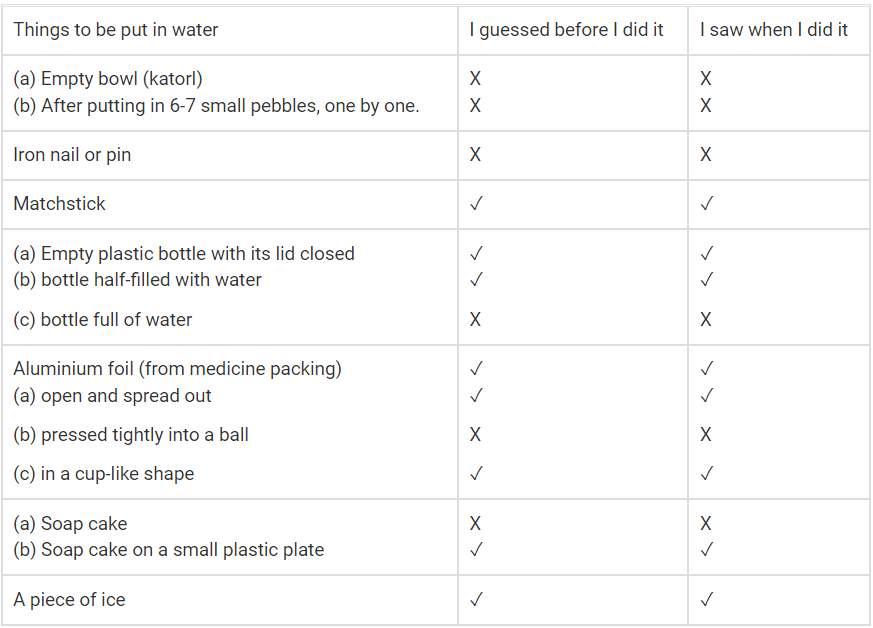
Q.2. Find out from the other groups which things float and which sank in the water?
Ans. A football, a piece of plastic ball etc. floats on water while a cricket ball, a spoon etc. sink.
Q.3. After Doing the Experiment, fill in the blanks
Ans:
1. The iron nail sank in the water but the Katori floated. I think this happened because the iron nail is denser than water, while the katori is lighter and has a shape that allows it to float.
2. The empty plastic bottle floated on the water. The bottle filled with water sank because it was heavier than the water.
3. The aluminium foil floated when it was spread out. When pressed tightly into a ball it sank. This may have happened because flat foil is light and spreads out, but when it’s in a ball, it becomes heavier and takes up less space, so it sinks in the water.
Is It Magic?
Q.1. Take some water in a glass. Put a lemon in it. Now keep putting salt in the water, half a spoon at a time. Were you able to float your lemon in water?Ans. Yes, the lemon floated in salty water.
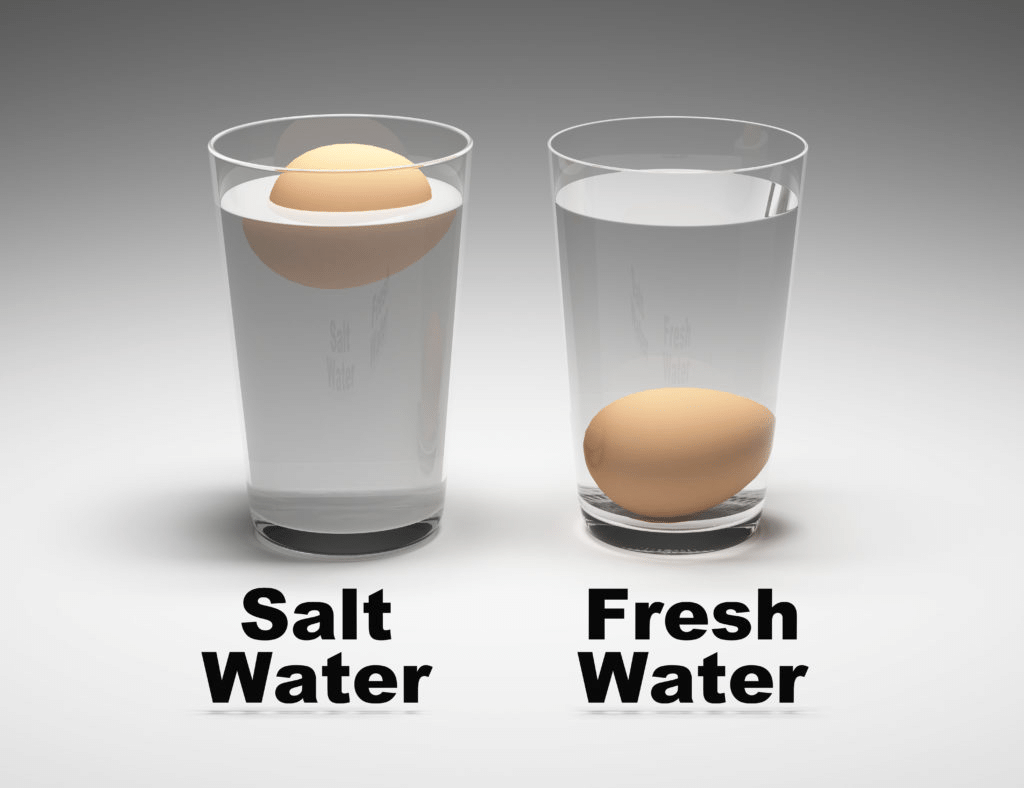 Egg in Fresh & Salt Water
Egg in Fresh & Salt Water
Q.2. What do you think, the lemon floated in salty water because….
Ans. The lemon floated in salty water because:
- The salt increases the density of the water: When you add salt to the water, it makes the water denser than plain water.
- The lemon is less dense than the salty water: Even though a lemon has some weight, its overall density is less than that of the saltwater, allowing it to float.
- Buoyancy: The upward force of the salty water is strong enough to keep the lemon afloat.
- So, the lemon can float in salty water due to the combined effects of increased water density and buoyancy.
What Dissolved and what did not?
Q.1. Suggest some ways to Hamid for Quickly Dissolving sugar.
Ans. To help Hamid dissolve sugar quickly, he can try the following methods:
- Continuously Stir the Mixture: By stirring the sugar and water together consistently, he helps to mix them better.
- Warm the Mixture: Heating the water slightly over a low flame can help dissolve sugar more quickly.
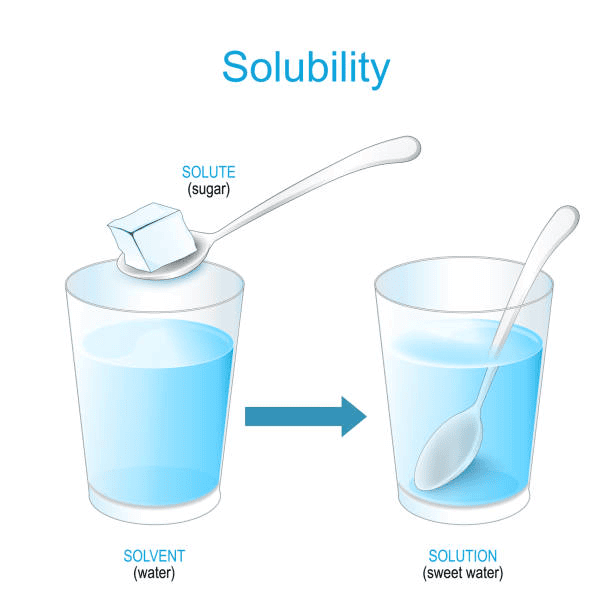 Sugar Solution
Sugar Solution
Do this Experiment
Q.1. Make groups of four friends. For the experiment, you will need 4-5 glasses or bowls or bowls and the things listed on the table. Take some water into each glass. Now try to dissolve one thing in one glass. Observe what happens and note it in the table.
Ans.
Tell
Q.1. Could you see the salt after it dissolved in water? If not, why?Ans. No, I could not see the salt because it got dissolved completely in the water, making it invisible.
Q.2. Does that mean that the water does not have salt? If it has, then where is the salt?
Ans. The water still contains salt even after it has dissolved. The salt is present in the water as individual salt molecules, which makes it not visible.
Q.3. What difference did you see in water with salt and water with chalk powder after keeping it for some time?
Ans. When salt dissolves in water, it makes the water look transparent. In contrast, chalk powder does not dissolve in water; instead, it settles at the bottom over time, making the water appear cloudy.
Q.4. Which of the two would you be able to separate from the water by straining with a cloth salt or chalk powder?
Ans. We can separate chalk powder by straining it with a cloth since it does not dissolve in water. However, salt dissolves in water, so we cannot separate it by straining.
Q.5. Do you think the oil dissolved in the water? Why do you think so?
Ans. No, the oil does not dissolve in water. It simply floats on the surface of the water because oil is less dense than water.
Racing Drops
Ayesha put two drops of oil on the lid of her tiffin box. Next to that, she put two drops of water and two drops of sugar solution. She tilted the lid. She saw some drops slid down quickly, while some were left behind. Racing Drops

Q.1. You also try to do the same and then which drop went ahead? Why did it slide faster?
Ans. The water drop went ahead. This happened because water does not stick to the tiffin box, while the sugar solution and oil drops stick to it.
Where Did the water go?
One day Ayesha’s mother put some water to boil on the stove for making tea. She got busy with something and forget about it. When she remembered and came to check, she found only a few drops of water left in the pan.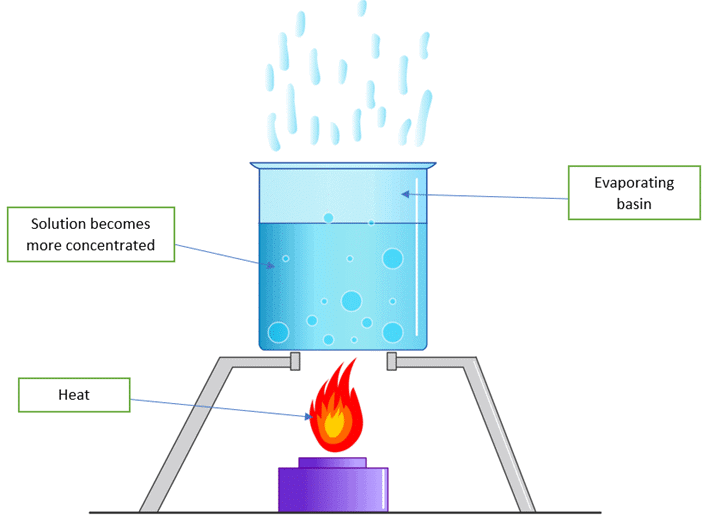 EvaporationQ.1. Think where did the water go?
EvaporationQ.1. Think where did the water go?
Ans. The water evaporated, which is why there was less water left in the pan.
Q.2. Why did Chittibabu and Chinnubabu keep their mango jelly in sun?
Ans. They kept their mango jelly in the sun to evaporate water from it, which helps in drying it.
Q.3. At your house, what things are made by drying in the sun?
Ans. At my house, we make eatables like mango jelly (aam papad), papad, bhaji (badi), and potato chips by drying them in the sun.
What we have Learned
Q.1. You have washed your handkerchief and you want to dry it quickly. What can you do?
Ans. To dry the washed handkerchief quickly:
- Squeeze Out Excess Water: Gently squeeze the handkerchief to remove as much water as possible without wringing it too hard.
- Spread it Out: Lay the handkerchief flat on a clean surface or a drying rack to allow air circulation.
- Dry in the Sun: Place the handkerchief in direct sunlight to speed up the drying process.
- Ironing: Use an iron on a low setting to press the handkerchief, which can help remove any remaining moisture quickly.
Q.2. What things do you put in water to make tea? Which of these things dissolve in water?
Ans. To prepare tea, you need:
- Sugar: Adds sweetness and dissolves completely in water.
- Tea Powder: Gives flavor but does not dissolve completely; it settles at the bottom.
- Milk: Adds creaminess and dissolves in water, changing the color and flavor of the tea.
Summary of Dissolving:
- Dissolves in Water: Sugar and milk.
- Does Not Dissolve: Tea powder (it settles at the bottom).
 Tea
Tea
Q.3. You have been given some mishri pieces (lumps of sugar). Suggest some ways to dissolve them quickly.
Ans. To dissolve mishri quickly:
- Crush the Mishri: Break the mishri into smaller pieces or crush it into powder to increase the surface area for dissolving.
- Stir Thoroughly: Add the crushed mishri to water and stir the mixture well to help it dissolve.
- Heat the Mixture: Gently heat the water to speed up the dissolving process, as warm water helps dissolve sugar faster.
|
37 videos|244 docs|41 tests
|
FAQs on NCERT Solutions for Class 5 EVS Chapter 7 - Experiments with Water
| 1. What are some common experiments that can be done with water? |  |
| 2. Why do some substances dissolve in water while others do not? |  |
| 3. How can we demonstrate that water can change states? |  |
| 4. What role does temperature play in the behavior of water? |  |
| 5. What are some key concepts learned from water experiments? |  |
















