Nernst Equation, Conductance, & Conductivity | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Nernst Equation |

|
| Conductance |

|
| Measurement of the Conductivity of Ionic Solutions |

|
| Change in Molar Conductivity: |

|
Nernst Equation
The Nernst equation is a fundamental equation in electrochemistry that relates the electromotive force (emf) of an electrochemical cell to the standard electrode potential, temperature, and concentration of the reactants and products involved.
The general form of the Nernst equation for a cell reaction is:
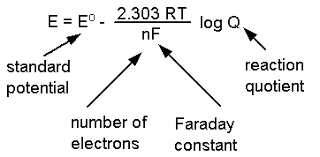
Walter Nernst derived a relation between cell potential and concentration or Reaction quotient.
ΔG = ΔGº RT ln Q ..........(i)
where, ΔG and ΔGº are free energy and standard free energy change, 'Q' is reaction quotient.
- ΔG = nFE and -ΔGº = nFEº
Thus from Eq. (i), we get - nFE = - nFEº + RT lnQ
At 25ºC, above equation may be written as E = Eº - 
Where 'n' represents number of moles of electrons involved in process.
E, Eº are e.m.f. and standard e.m.f. of the cell respectively.
In general, for a redox cell reaction involving the transference of n electrons
aA + bB → cC + dD, the EMF can be calculated as: ECell = EºCell - |
Thermodynamic Treatment of Nernst Equation
(i) Prediction and feasibility of spontaneity of a cell reaction:
Let us see whether the cell (Daniell) is feasible or not; i.e. whether Zinc will displace copper or not.
Zn | (s) | ZnSO4(sol) || CuSO4(sol) | Cu(s)


= 0.34 - (- 0.76) = +1.10 volt
Since E0 = +ve, hence the cell will be feasible and zinc will displace copper from its salt solution. In the other words zinc will reduce copper.
(ii) Determination of equilibrium constant form Nernst Equation:
We know, that
E = E0 - ..........(1)
..........(1)
At equilibrium, the cell potential is zero because cell reactions are balanced, i.e. E = 0
From Eq. (i), we have
0 = E0 - or Keq = anti
or Keq = anti 
(iii) Heat of Reaction inside the cell :
Let n Faraday charge flows out of a cell of e.m.f. E, then
- ΔG = nFE ..........(i)
Gibbs Helmholtz equation (from thermodynamics) may be given as,
ΔG = ΔH + T  ..........(ii)
..........(ii)
From Eqs. (i) and (ii), we have
- nFE = ΔH +T  = DH - nFT
= DH - nFT
ΔH = - nFE + nFT
(iv) Entropy change inside the cell :
We know that
G = H - TS or ΔG = ΔH - TΔS ..........(i)
where ΔG = Free energy change ; ΔH = Enthalpy change and ΔS = entropy change.
According to Gibbs Helmholtz equation,
ΔG = ΔH +T ............(ii)
............(ii)
From Eqs. (i) and (ii), we have
- TΔS = T or ΔS = -
or ΔS = -
or ΔS = nF
where  is called temperature coefficient of cell e.m.f.
is called temperature coefficient of cell e.m.f.
Different types of half-cells and their reduction potential
(1) Gas - Ion Half Cell :
In such a half cell, an inert collector of electrons, platinum or graphite is in contact with gas and a solution containing a specified ion. One of the most important gas-ion half cell is the hydrogen-gas-hydrogen ion half cell. In this cell, purified H2 gas at a constant pressure is passed over a platinum electrode which is in contact with an acid solution.
H (aq) e- 1/2 H2
1/2 H2

(2) Metal-Metal Ion Half Cell :
This type of cell consist of a metal M is contact with a solution containing Mn ions.
Mn (aq) + ne-  M(s)
M(s)

(3) Metal-Insoluble Salt-Anion Half Cell :
In this half cell, a metal coated with its insoluble salt is in contact with a solution containing the anion of the insoluble salt. eg.-Silver-Silver Chloride Half Cell :
This half cell is represented as Cl-/AgCl/Ag. The equilibrium reaction that occurs at the electrode is
AgCl(s) + e- Ag+(s) + Cl-(aq)
Ag+(s) + Cl-(aq)
 ,
, 
(4) Oxidation-Reduction Half Cell :
This type of half cell is made by using an inert metal collector, usually platinum, immersed in a solution which contains two ions of the same element in different states of oxidation. eg. Fe2 - Fe3 half cell.
Fe3+ (aq) + e- Fe2+ (aq)
Fe2+ (aq)

(5) Concentration Cell :
The cells in which electrical current is produced due to transport of a substance from higher to lower concentration. Concentration gradient may arise either in electrode material or in electrolyte. Thus there are two types of concentration cell.
(i) Electrode Gas Concentration Cell :
Pt, H2(P1)|H+ (C)|H 2(P2), Pt
Here, hydrogen gas is bubbled at two different partial pressures at electrode dipped in the solution of same electrolyte.
Cell Process : 1/2H2(p1) → H (c) e- (Anode process)

E = 
or E = -
At 25ºC, E = -
For spontanity of such cell reaction p1 > p2
(ii) Electrolyte concentration cells:
Zn(s) |ZnSO4(C1)||ZnSO4(C 2) | Zn(s)
In such cells, concentration gradient arise in electrolyte solutions. Cell process may be given as,
Zn(s) → Zn2 (C1) + 2e- (Anodic process)
 (Over all process)
(Over all process)
From Nernst equation, we have
 or
or 
For spontaneity of such cell reaction, C2 > C1 .
Conductance
Conductance refers to the ability of a substance to conduct electricity. It's a measure of how easily electric current flows through a material. It is influenced by various factors such as the concentration of ions in a solution, the mobility of ions, and the temperature of the system.
In solutions, conductance primarily depends on the concentration of ions dissolved in the solution.
- Strong electrolytes, which completely dissociate into ions, have high conductance because they provide more ions for the electric current to flow through.
- Weak electrolytes, which partially dissociate, have lower conductance.
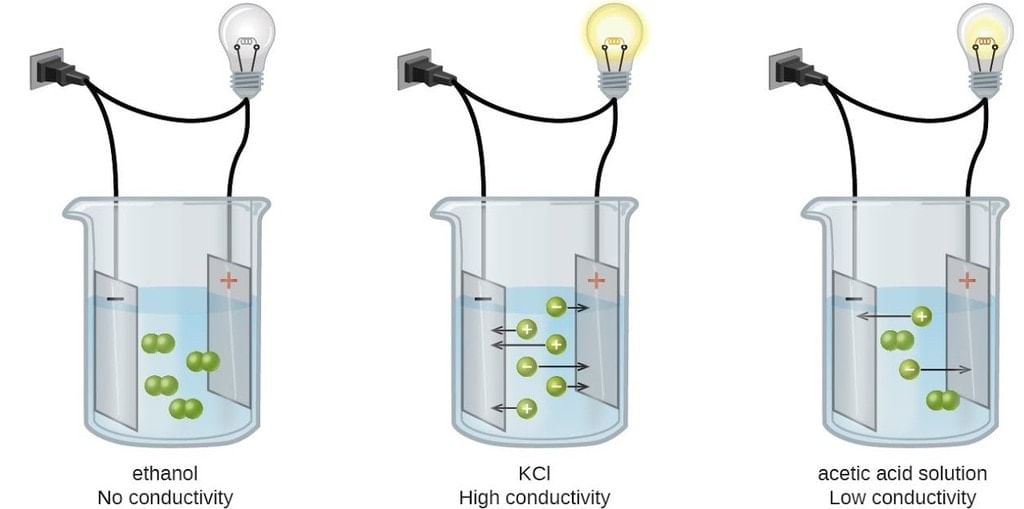 Conductance/ Conductivity of Different Electrolytic Solutions
Conductance/ Conductivity of Different Electrolytic Solutions
Conductivity
Conductivity refers to the ability of a material to conduct electrical current. It's a measure of how easily electrical charges can flow through a substance. Materials can be classified as conductors, insulators, or semiconductors based on their conductivity properties.
 |
Test: Electrochemistry - 2
|
Start Test |
Measurement of the Conductivity of Ionic Solutions
Both metallic and electrolytic conductors obey Ohm's law i.e. V = IR
where
- V = Potential difference in volt
- I = Current in ampere
- R = resistance in Ohm
We know, resistance is directly proportional to length of conductor and inversely proportional to cross sectional area of the conductor.
 Specific resistance is the resistance of a conductor having lengths of 1 cm and cross sectional area of 1 cm2.
Specific resistance is the resistance of a conductor having lengths of 1 cm and cross sectional area of 1 cm2.
Unit of R is ohm and unit of specific resistance is ohm cm
Note: Reciprocal of resistance is called as conductance and reciprocal of specific resistance is called as specific conductance.

where C = conductance ohm-1; K = specific conductance ohm-1 cm-1.
Ohm and siemens are other units of conductance.

Specific conductance = Cell constant x Conductance.
Specific Conductance is Conductance of 1 cm3 of an electrolyte solution.
In case of electrolytic solution, the specific conductance is defined as the conductance of a solution of definite concentration enclosed in a cell having two electrodes of unit area separated by 1 cm apart.
- Equivalent Conductance
Equivalent conductance is the conductance of an electrolyte solution containing 1 gm equivalent of electrolyte. It is denoted by ^.
^ = K × V
( ^ = ohm-1 cm-1 × cm3 = ohm-1 cm2)
Usually concern ration of electrolyte solution is expresses as C gm equivalent per litre.
Thus, V =
{Volume having 1 gm equivalent electrolyte in the solution} Thus, ^ = K × .
. - Molar Conductance
Molar conductance may be defined as conductance of an electrolyte solution having 1 gm mole electrolyte in a litre. It is denoted by ^m.
^m = K × V
Usually concentration of electrolyte solution is expressed as 'M' gm mole electrolyte per litre.
Thus, V =
Hence, ^m = K ×
| Note: Relation between ^ and ^m = ^m = n × ^ |
Measurement of the Conductivity of Ionic Solutions
Molar Conductivity
The conductivity produced by dissolving 1 gram-mole of an electrolyte placed between two large electrodes at one centimeter apart is called as molar conductivity.
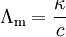
k is the measured conductivity c is the electrolyte solution
Units of Molar Conductivity
What is Specific Conductivity?
Specific conductivity or conductivity of an electrolytic solution at any given concentration is the conductance of unit volume of solution. It is the conductance when kept between two platinum electrodes with a unit area of cross-section.
The electrodes are at a distance of unit length.
Conductivity decreases with a decrease in concentration as the number of ions per unit volume that carry the current in a solution decrease on dilution.
The molar conductivity of a solution at a given concentration is the conductance of volume V of the solution containing one mole of electrolyte kept between two electrodes with an area of cross section A and distance of unit length.
Ʌm = К/c
- c = concentration in moles per volume
- К = specific conductivity
- Ʌm = molar conductivity
As the solution contains only one mole of electrolyte, the above equation can be modified as:
Ʌm = КV
Variation of Conductivity and Molar Conductivity with Concentration
 |
Download the notes
Nernst Equation, Conductance, & Conductivity
|
Download as PDF |
Change in Molar Conductivity:
Molar conductivity increases with a decrease in concentration. This happens because the total volume, V, of the solution containing one mole of electrolyte also increases. Upon dilution, the concentration decreases.
Furthermore, when the concentration approaches zero, the molar conductivity of the solution is known as limiting molar conductivity, Ë°m.
Variation of molar conductivity with concentration is different for both, strong and weak electrolytes.
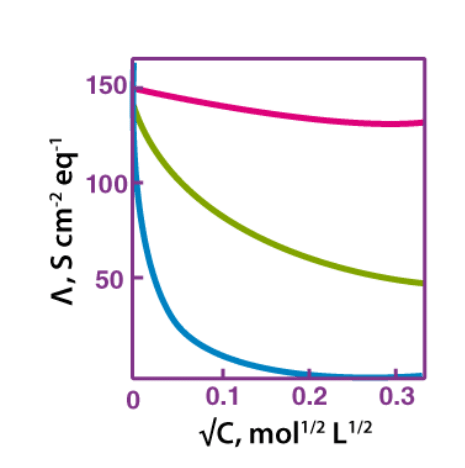 Variation of Molar Conductivity with Concentration
Variation of Molar Conductivity with Concentration
(i) Variation of Molar Conductivity with Concentration of Strong Electrolytes:
- For strong electrolytes, the molar conductivity increases slowly with the dilution. The plot of the molar conductivity and c1/2 is a straight line having y-intercept equal to Ë°m. The value of limiting molar conductivity, Ë°m can be determined from the graph or with the help of Kohlrausch law.
- For strong electrolytes, the molar conductivity increases slowly with the dilution. Thus, the plot of the molar conductivity and c1/2 is a straight line having y-intercept equal to Ë°m. The general equation for the plot is:
- Ʌm = Ë°m − Ac1/2
Where −A is a constant equal to the slope of the line. Furthermore, the value of “A” for a given solvent depends on the type of electrolyte at a particular temperature. Hence, it differs from solution to solution.
(ii) Variation of Molar Conductivity with Concentration for Weak Electrolytes:
- For weak electrolytes, the graph plotted between molar conductivity and c1/2 (where c is the concentration) is not a straight line.
- This is because weak electrolytes have lower molar conductivities and lower degree of dissociation at higher concentrations which increases steeply at lower concentrations.
- Hence, we use the Kohlrausch law of independent migration of ions for determining to limit molar conductivity, Ëm° of weak electrolytes.
Variation of Conductivity & Molar Conductivity with Concentration Graphically
A plot of ^m vs as found experimentally is as shown below graphically.

- The ^m vs
 plot of strong electrolyte being linear it can be extrapolated to zero concentration.
plot of strong electrolyte being linear it can be extrapolated to zero concentration. - Thus, ^m values of the solution of the test electrolyte are determined at various concentrations the concentrations should be as low as good.
- ^m values are then plotted against
 when a straight line is obtained. This is the extrapolated to zero concentration. The point where the straight line intersects ^m axis is ^ºm of the strong electrolyte.
when a straight line is obtained. This is the extrapolated to zero concentration. The point where the straight line intersects ^m axis is ^ºm of the strong electrolyte. - However, the plot in the case weak electrolyte being non linear, shooting up suddenly at some low concentration and assuming the shape of a straight line parallel to ^m axis. Hence extrapolation in this case is not possible.
- Thus, ^0 of a weak electrolyte cannot be determined experimentally. It can, however, be done with the help of Kohlrausch's law to be discussed later.
|
108 videos|287 docs|122 tests
|
FAQs on Nernst Equation, Conductance, & Conductivity - Chemistry Class 12 - NEET
| 1. What is the Nernst Equation? |  |
| 2. How is conductance measured in the context of ionic solutions? |  |
| 3. What is the significance of measuring the conductivity of ionic solutions? |  |
| 4. How does molar conductivity change with respect to the Nernst Equation? |  |
| 5. How are Nernst Equation, Conductance, and Conductivity related in the context of NEET exam preparation? |  |






















