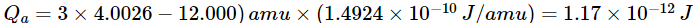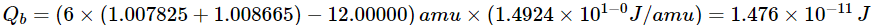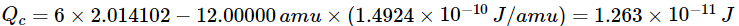Nuclear Fusion | Chemistry for ACT PDF Download
Introduction
During the conversion of extremely light atomic nuclei into heavier ones, a significant amount of energy is released, known as fusion. The primary source of energy in the sun is a fusion reaction involving four hydrogen nuclei that combine to form one helium nucleus and two positrons. This reaction represents a net outcome of a complex sequence of events.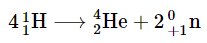 When four hydrogen nuclei combine to form a helium nucleus, the resulting helium nucleus has a slightly lower mass, approximately 0.7% less, compared to the combined mass of the four hydrogen nuclei. This lost mass is converted into energy during the fusion process. Each mole of
When four hydrogen nuclei combine to form a helium nucleus, the resulting helium nucleus has a slightly lower mass, approximately 0.7% less, compared to the combined mass of the four hydrogen nuclei. This lost mass is converted into energy during the fusion process. Each mole of 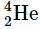 produced in this fusion reaction yields around 3.6 × 1011 kJ of energy. This energy output is relatively higher than the energy released by the nuclear fission of one mole of U-235, which amounts to approximately 1.8 × 1010 kJ. Furthermore, it is over three million times greater than the energy obtained from the combustion of one mole of octane through a chemical reaction, which results in 5471 kJ of energy.
produced in this fusion reaction yields around 3.6 × 1011 kJ of energy. This energy output is relatively higher than the energy released by the nuclear fission of one mole of U-235, which amounts to approximately 1.8 × 1010 kJ. Furthermore, it is over three million times greater than the energy obtained from the combustion of one mole of octane through a chemical reaction, which results in 5471 kJ of energy.
It has been determined that the nuclei of the heavy isotopes of hydrogen, a deuteron,  undergo fusion at extremely high temperatures (thermonuclear fusion). They form a helium nucleus and a neutron:
undergo fusion at extremely high temperatures (thermonuclear fusion). They form a helium nucleus and a neutron: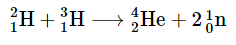 This change proceeds with a mass loss of 0.0188 amu, corresponding to the release of 1.69 × 109 kilojoules per mole of
This change proceeds with a mass loss of 0.0188 amu, corresponding to the release of 1.69 × 109 kilojoules per mole of 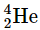 formed. The very high temperature is necessary to give the nuclei enough kinetic energy to overcome the very strong repulsive forces resulting from the positive charges on their nuclei so they can collide.
formed. The very high temperature is necessary to give the nuclei enough kinetic energy to overcome the very strong repulsive forces resulting from the positive charges on their nuclei so they can collide.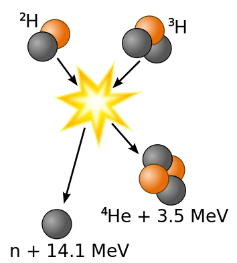
Figure 1: Fusion of deuterium with tritium creating helium-4, freeing a neutron, and releasing 17.59 MeV of energy, as an appropriate amount of mass changing forms to appear as the kinetic energy of the products, in agreement with kinetic E = Δmc2, where Δm is the change in rest mass of particles.
The primary source of energy in stars, including our Sun, is a fusion process. It was recognized in the 20th century that the energy released through nuclear fusion reactions is responsible for the sustained heat and light emissions from these celestial bodies. As nuclei fuse together within a star, starting from an initial abundance of hydrogen and helium, energy is generated, and new nuclei are synthesized as byproducts of this fusion process. In the Sun specifically, the main energy-producing reaction is the fusion of hydrogen nuclei to form helium. This occurs at an extremely high temperature of around 14 million kelvin in the solar core. The outcome of this fusion process is the fusion of four protons, resulting in the creation of one alpha particle, the emission of two positrons and two neutrinos (which convert some protons into neutrons), and the release of energy (refer to Figure 2).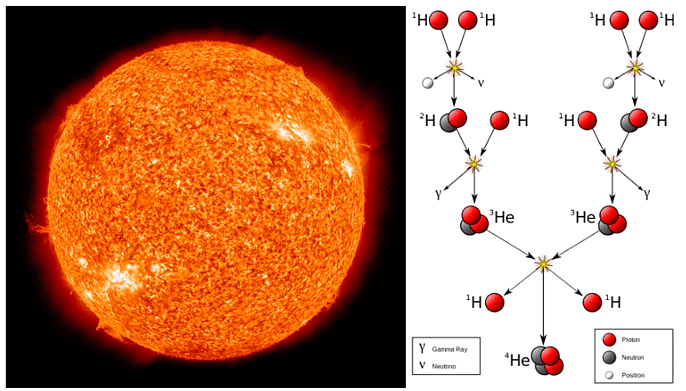
Figure 2: (left) The Sun is a main-sequence star, and thus generates its energy by nuclear fusion of hydrogen nuclei into helium. In its core, the Sun fuses 620 million metric tons of hydrogen each second. (right) The proton-proton chain dominates in stars the size of the Sun or smaller.
Example: Calculate the energy released in each of the following hypothetical processes.
(a) 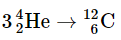
(b) 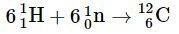
(c) 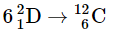
(a)
(b)
(c)
Fusion of He to give C releases the least amount of energy, because the fusion to produce He has released a large amount. The difference between the second and the third is the binding energy of deuterium. The conservation of mass-and-energy is well illustrated in these calculations. On the other hand, the calculation is based on the conservation of mass-and-energy.
Nuclear Reactors
To initiate useful fusion reactions, extremely high temperatures of approximately 15,000,000 K or higher are required. At such elevated temperatures, molecules break apart into individual atoms, and the atoms become ionized, forming a state of matter called plasma. These extreme conditions are prevalent in numerous locations across the universe, as they are responsible for powering stars through fusion processes. Notably, humans have already devised methods to generate temperatures capable of supporting fusion on a large scale, as demonstrated in thermonuclear weapons. A thermonuclear weapon, such as a hydrogen bomb, consists of a nuclear fission bomb that, upon detonation, releases sufficient energy to attain the extremely high temperatures necessary for fusion reactions to occur.
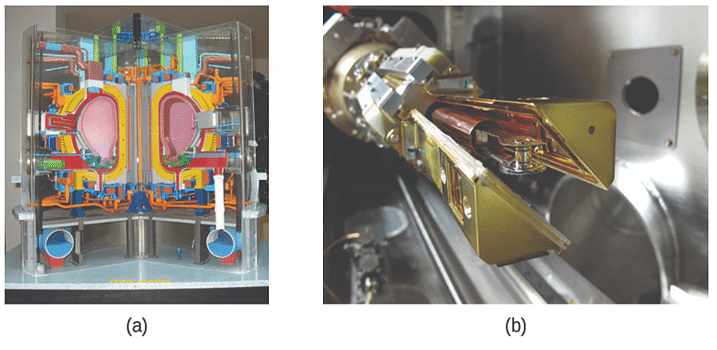
Figure 3: (a) This model is of the International Thermonuclear Experimental Reactor (ITER) reactor. Currently under construction in the south of France with an expected completion date of 2027, the ITER will be the world’s largest experimental Tokamak nuclear fusion reactor with a goal of achieving larg\times 10^{scale sustained energy production.
(b) In 2012, the National Ignition Facility at Lawrence Livermore National Laboratory briefly produced over 500,000,000,000 watts (500 terawatts, or 500 TW) of peak power and delivered 1,850,000 joules (1.85 MJ) of energy, the largest laser energy ever produced and 1000 times the power usage of the entire United States in any given moment. Although lasting only a few billionths of a second, the 192 lasers attained the conditions needed for nuclear fusion ignition. This image shows the target prior to the laser shot.
An alternative and highly advantageous method for inducing fusion reactions is through the use of a fusion reactor. Unlike in thermonuclear weapons, the goal of a fusion reactor is to achieve controlled fusion reactions involving light atomic nuclei. Since conventional solid materials cannot withstand the extreme temperatures involved, it is not feasible to employ mechanical devices to contain the plasma where fusion reactions take place.
Presently, there are two prominent techniques that are extensively researched for containing plasma at the required density and temperature for fusion reactions: magnetic field confinement and focused laser beam confinement (refer to Figure 3). Numerous large-scale projects are dedicated to accomplishing a significant scientific milestone: igniting hydrogen fuel in a way that generates more energy than the energy supplied to attain the extremely high temperatures and pressures essential for fusion. As of the time of writing, there are no operational self-sustaining fusion reactors worldwide, although controlled fusion reactions on a small scale have been achieved for very brief durations.
|
110 videos|130 docs|117 tests
|