Number of Atoms in a Unit Cell | Physical Chemistry PDF Download
Q.1. The total number of atoms in one unit cell of face-centered unit cubic cell is ______ atoms.
(a) 2
(b) 6
(c) 4
(d) 8
Correct Answer is Option (b)
In face-centered cubic unit cell, atoms are present at each of the corners and at the centre of the face of the cube.
8 corners ×of an atom = 1
6 faces ×of an atom = 3
Thus, the total number of atoms in face-centered cubic unit cell: 1+3=4 atoms.
Q.2. The total number of atoms in one unit cell of primitive unit cubic cell is ______ atom(s).
(a) 1
(b) 8
(c) 4
(d) 2
Correct Answer is Option (a)
In primitive cubic unit cell, atoms are present only at the corner of the cell. Thus, in actual, only
of an atom (or ion or molecule) belongs to a particular unit cell. Hence, the total number of atoms in primitive cubic unit cell = 8 ×
= 1 atom.
Q.3. The total number of atoms in one unit cell of body-centered unit cubic cell is ______ atoms.
(a) 4
(b) 3
(c) 8
(d) 2
Correct Answer is Option (d)
In body-centered cubic unit cell, one atom is present at each of the corners and one atom at its body center.
8 corners ×of an atom = 1
1 body-centered atom
Thus, the total number of atoms in body-centered cubic unit cell: 1+1=2 atoms.
Q.4. In primitive unit cubic cell, only _______ of an atom (or ion or molecule) belongs to a particular unit cell.
(a)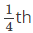
(b)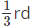
(c)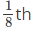
(d) 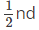
Correct Answer is Option (c)
In primitive unit cubic cell, each atom at the corner is shared between 8 adjacent unit cells. Thus, only
of an atom (or ion or molecule) belongs to a particular unit cell.
|
90 videos|144 docs|67 tests
|
FAQs on Number of Atoms in a Unit Cell - Physical Chemistry
| 1. How can I determine the number of atoms in a unit cell? |  |
| 2. What is the relationship between lattice points and atoms in a unit cell? |  |
| 3. How does the crystal structure affect the number of atoms in a unit cell? |  |
| 4. Can the number of atoms in a unit cell vary for the same crystal structure? |  |
| 5. How does the number of atoms in a unit cell affect the properties of a material? |  |
















