Number of Stereoisomers & Enantiomeric Excess | Organic Chemistry PDF Download
Number of Stereisomers in an organic compound.
For a molecule (asymmetrical), with n stereogenic centers, the maximum number of stereoisomers is 2n. Let us now consider the stereoisomers of 2,3-dibromobutane. Since this molecule has two stereogenic centers, the maximum number of stereoisomers is 4.
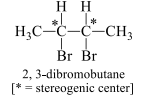
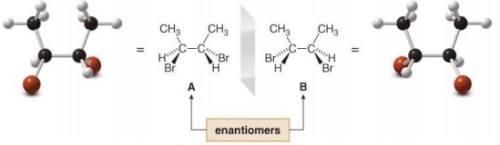
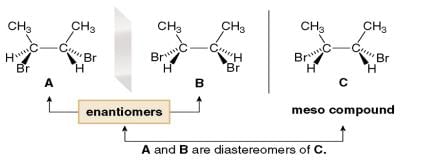
To find all the stereoisomers of 2,3-dibromobutane, arbitrarily add the H, Br, and CH3 groups to the stereogenic centers, forming one stereoisomer A, and then draw its mirror image, B.
To find the other two stereoisomers if they exist, switch the position of two groups on one stereogenic center of one enantiomer only. In this case, switching the positions of H and Br on one stereogenic center of A forms C, which is different from both A and B.
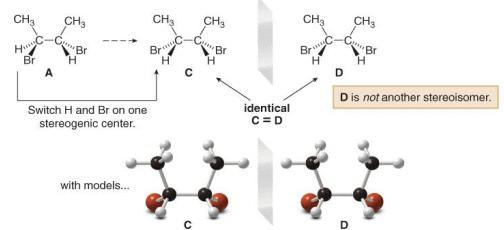
A Meso compound is an achiral compound that contains tetrahedral stereogenic centers. C is a meso compound. Compound C contains a plane of symmetry, and is achiral. Meso compounds generally contain a plane of symmetry so that they possess two identical halves.
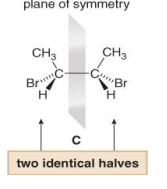
Because one stereoisomer of 2,3-dibromobutane is superimposable on its mirror image, there are only three stereoisomers, not four.
Enantiomeric Excess
Enantiomeric excess (optical purity) is a measurement of how much one enantiomer is present in excess of the racemic mixture. It is denoted by the symbol ee.
ee = % of one enantiomer - % of the other enantiomer
Consider the following example—If a mixture contains 75% of one enantiomer and 25% of the other, the enantiomeric excess is 75% - 25% = 50%. Thus, there is a 50% excess of one enantiomer over the racemic mixture. The enantiomeric excess can also be calculated if the specific rotation [α] of a mixture and the specific rotation [α] of a pure enantiomer are known.
ee = ([α] mixture/[α] pure enantiomer) x 100
- Since enantiomers have identical physical properties, they cannot be separated by common physical techniques like distillation.
- Diastereomers and constitutional isomers have different physical properties, and therefore can be separated by common physical techniques.
Optical Rotation
- The chemical and physical properties of two enantiomers are identical except for how they interact with plane-polarized light.
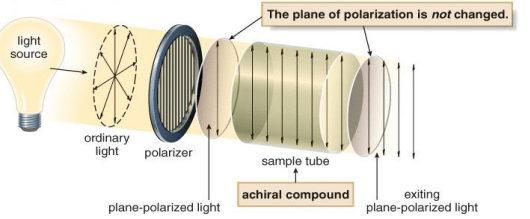
- Plane-polarized light (PPL) is light that has an electric vector that oscillates in a single plane.Plane-polarized light arises from passing ordinary light through a polarizer.
- A polarimeter is an instrument that allows polarized light to travel through a sample tubecontaining an organic compound. It permits the measurement of the degree to which an organiccompound rotates plane-polarized light.
With achiral compounds, the light that exits the sample tube remains unchanged. A compound that does not change the plane of polarized light is said to be optically inactive.
With chiral compounds, the plane of the polarized light is rotated through an angle α. The angle α is measured in degrees (°), and is called the observed rotation. A compound that rotates polarized light is said to be optically active.
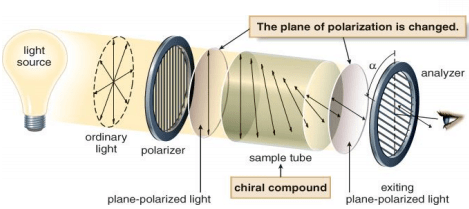
The rotation of polarized light can be clockwise or anticlockwise.
- If the rotation is clockwise (to the right of the noon position), the compound is called dextrorotatory. The rotation is labeled d or (+).
- If the rotation is counterclockwise, (to the left of noon), the compound is called levorotatory. The rotation is labeled l or (–).
- Two enantiomers rotate plane-polarized light to an equal extent but in opposite directions. Thus, if enantiomer A rotates polarized light +5°, the same concentration of enantiomer B rotates it –5°.
- No relationship exists between R and S prefixes and the (+) and (–) designations that indicate optical rotation.
Specific rotation:
It is a standardized physical constant for the amount that a chiral compound rotates
plane-polarized light. Specific rotation is denoted by the symbol [α] and defined using a specific sample tube length (l, in dm), concentration (c in g/mL), temperature (250C) and wavelength (589 nm).


Racemic Mixture
An equal amount of two enantiomers is called a racemic mixture or a racemate. A racemic mixture is optically inactive. Because two enantiomers rotate plane-polarized light to an equal extent but in opposite directions, the rotations cancel, and no rotation is observed.
|
44 videos|102 docs|52 tests
|
FAQs on Number of Stereoisomers & Enantiomeric Excess - Organic Chemistry
| 1. What is the definition of stereoisomers? |  |
| 2. How do you determine the number of stereoisomers in a molecule? |  |
| 3. What is enantiomeric excess (ee)? |  |
| 4. How is enantiomeric excess calculated? |  |
| 5. Can enantiomeric excess be higher than 100%? |  |

















