Partial Molar Property | Physical Chemistry PDF Download
A partial molar property is a thermodynamic quantity which describes the variation of an extensive property of a solution or mixture with changes in the molar composition of the mixture at constant temperature and pressure. It is the partial derivative of the extensive property with respect to the amount (number of moles) of the component of interest. Every extensive property of a mixture has a corresponding partial molar property.
Definition
The partial molar volume is broadly understood as the contribution that a component of a mixture makes to the overall volume of the solution. However, there is more to it than this:
When one mole of water is added to a large volume of water at 25°C, the volume increases by 18 cm3. The molar volume of pure water would thus be reported as 18 cm3 mol−1. However, addition of one mole of water to a large volume of pure ethanol results in an increase in volume of only 14 cm3. The reason that the increase is different is that the volume occupied by a given number of water molecules depends upon the identity of the surrounding molecules. The value 14 cm3 is said to be the partial molar volume of water in ethanol.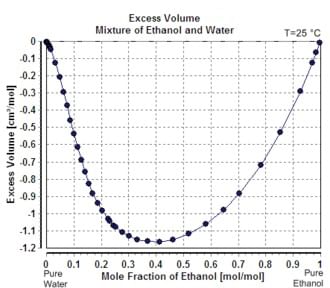
Water and ethanol always have negative excess volumes when mixed, indicating the partial molar volume of each component is less when mixed than its molar volume when pure.
In general, the partial molar volume of a substance X in a mixture is the change in volume per mole of X added to the mixture.
The partial molar volumes of the components of a mixture vary with the composition of the mixture, because the environment of the molecules in the mixture changes with the composition. It is the changing molecular environment (and the consequent alteration of the interactions between molecules) that results in the thermodynamic properties of a mixture changing as its composition is altered
If, by Z, one denotes a generic extensive property of a mixture, it will always be true that it depends on the pressure P, temperature T, and the amount of each component of the mixture (measured in moles, n). For a mixture with q components, this is expressed as
Z = Z(T,P,n1,n2,...)
Now if temperature T and pressure P are held constant, Z=Z(n1,n2,...) is a homogeneous function of degree 1, since doubling the quantities of each component in the mixture will double Z. More generally, for any λ:
Z(λn1,λn2,...) = λZ(n1,n2,...)
By Euler's first theorem for homogeneous functions, this implies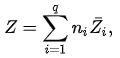
where 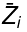 is the partial molar Z of component i defined as:
is the partial molar Z of component i defined as: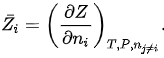
By Euler's second theorem for homogeneous functions, 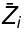 is a homogeneous function of degree 0 which means that for any λ:
is a homogeneous function of degree 0 which means that for any λ: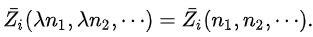
In particular, taking λ =1/nT where nT=n1 +n2 + .... , one has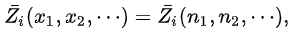
where 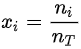 is the concentration expressed as the mole fraction of component i. Since the molar fractions satisfy the relation
is the concentration expressed as the mole fraction of component i. Since the molar fractions satisfy the relation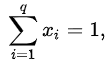
the xi are not independent, and the partial molar property is a function of only q-1 mole fractions: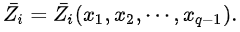
The partial molar property is thus an intensive property - it does not depend on the size of the system. The partial volume is not the partial molar volume.
Applications
Partial molar properties are useful because chemical mixtures are often maintained at constant temperature and pressure and under these conditions, the value of any extensive property can be obtained from its partial molar property. They are especially useful when considering specific properties of pure substances (that is, properties of one mole of pure substance) and properties of mixing (such as the heat of mixing or entropy of mixing). By definition, properties of mixing are related to those of the pure substances by: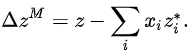
Here * denotes a pure substance, M the mixing property, and z corresponds to the specific property under consideration. From the definition of partial molar properties,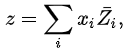
substitution yields: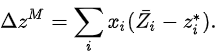
So from knowledge of the partial molar properties, deviation of properties of mixing from single components can be calculated.
Relationship to thermodynamic potentials
Partial molar properties satisfy relations analogous to those of the extensive properties. For the internal energy U, enthalpy H, Helmholtz free energy A, and Gibbs free energy G, the following hold: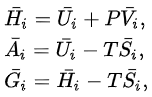
where P is the pressure, V the volume, T the temperature, and S the entropy.
Differential form of the thermodynamic potentials
The thermodynamic potentials also satisfy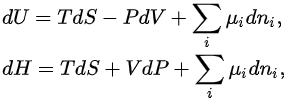
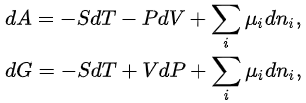
where μi is the chemical potential defined as (for constant nj with j≠i):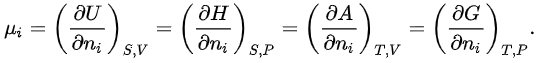
This last partial derivative is the same as 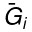 , the partial molar Gibbs free energy. This means that the partial molar Gibbs free energy and the chemical potential, one of the most important properties in thermodynamics and chemistry, are the same quantity. Under isobaric (constant P) and isothermal (constant T ) conditions, knowledge of the chemical potentials, μi(x1,x2,...,xm) yields every property of the mixture as they completely determine the Gibbs free energy.
, the partial molar Gibbs free energy. This means that the partial molar Gibbs free energy and the chemical potential, one of the most important properties in thermodynamics and chemistry, are the same quantity. Under isobaric (constant P) and isothermal (constant T ) conditions, knowledge of the chemical potentials, μi(x1,x2,...,xm) yields every property of the mixture as they completely determine the Gibbs free energy.
|
90 videos|144 docs|67 tests
|





















