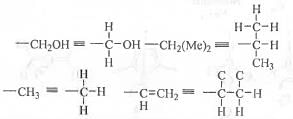Question for Past Year Question: Stereochemistry Of Organic Molecules
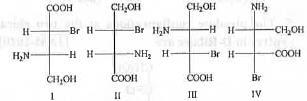
Try yourself:Among the following compounds, the pair of enantiomers is
[2017]
Explanation
Two compounds are termed as enantiomers which are mirror image to each other. Let us analyze the following compounds :
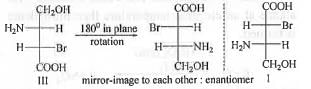
Hence, among the given compounds, the pair of enantiomers is : I and III.
Hence, the correct answer is : B.
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
Try yourself:Catalytic hydrogenation of the following compound produces saturated hydrocarbon(s). The no. of stereoisomer(s) formed is
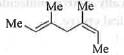
[2017]
Explanation
Catalytic hydrogenation of the given compound produces one fully saturated compound which contains two chiral centre. Hence, the no. of isomers should be : 2n = 22 = 4. But, due to the presence of plane of symmetry, the no. of optically inactive meso form will be : 1. Hence, the no. of optically active form will be 2. Hence, the total no. of stereoisomer(s) are : (1+2) = 3. These are shown below:
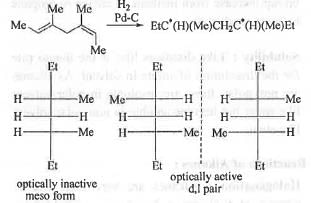
Hence, the correct answer is : C.
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
Try yourself:The compounds having C3-axis of symmetry are
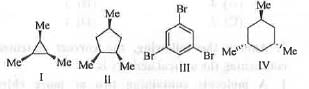
[2017]
Explanation
Compounds II and IV has C2 -axis of symmetry but these are devoid of C3-axis. But, compounds I and III contains C2 as well as C3-axis of symmetry. These are shown below :
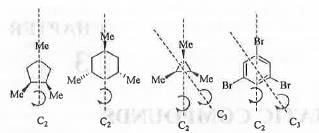
Hence, the correct answer is : C.
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
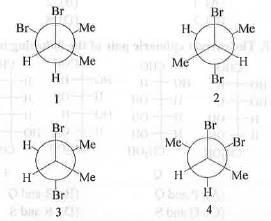
Try yourself:Among the following, the most stable conformations of meso-2,3-dibromobutane is
[2016]
Explanation
Methyl groups are bulky in nature. Similarly, bromide groups are also very bulky in nature. Hence, the most stable conformations of meso-2,3- dibromobutane is one which contains two bulky methyl groups at farthest distance and two bulky bromide groups are also anti to each other.
Now, methyl groups are slightly positively polarized while bromide groups are negatively polarized. Hence, when these groups are close to each other, there will be hydrophobic interactions which will make the molecule stable.
Both these conditions are full-filled in the conformation 2. Hence, the most stable conformation is : 2.
Hence, the correct answer is : B .
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
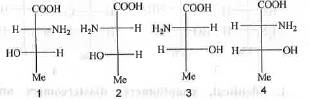
Try yourself:The structure of (2S, 3R)-2-amino-3-hydroxy butanoic acid is
[2015]
Explanation
According to the C.I.P. rule the priority order of the groups : -NH2 > -COOH > -CH(OH)(Me) > -H at C-2 centre and -OH > -CH(NH2)(COOH) > -Me > -H at C-3 centre.
Hence, the correct answer is : C.
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
Try yourself:Which among the following molecules is chiral?
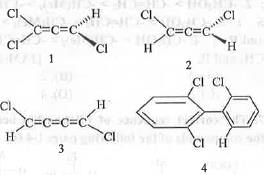
[2014]
Explanation
Compounds 1 and 2 are examples of substituted allene. In case 1, due to the presence of plane of symmetry (σ), it is optically inactive and hence, will acts as achiral molecule.
Compound 3 is an example of odd cumulated diene. Hence, it has centre of symmetry (i). Due to the presence of i, it will also become achiral.
In case of 4, as steric crowding is not strong enough to stop the free rotation about the C-C bond and hence, it will exist as a pair of inseparable compounds. Hence, compound 4 is achiral.
But, in case of substituted allene 2, only C2 is present as the symmetry element. Hence, it is optically active. Hence, it is chiral.
Hence, the correct answer is : B.
Report a problem
*Multiple options can be correct
Question for Past Year Question: Stereochemistry Of Organic Molecules
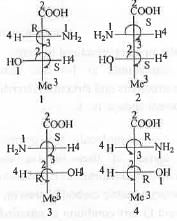
Try yourself:The correct epimeric pair of the following is
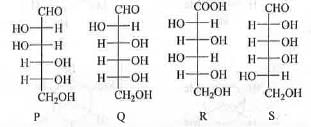
Explanation
In case of sugar molecule, two compounds are said to be epimer if they involve change in conformation in any asymmetric carbon atom when more than one asymmetric carbon is present.
In case of P and Q this condition is satisfied. Hence, P and Q are epimer.
Similarly, in case of Q and R, this condition is also satisfied. Hence, Q and R are also epimer.
Hence, the correct answer is : (A, B).
Question for Past Year Question: Stereochemistry Of Organic Molecules
Try yourself:The correct sequence of relationship between the compounds of the following pairs 1-4 is
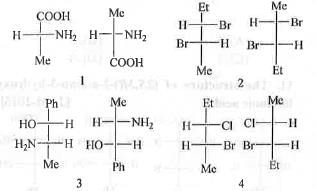
1. identical, enantiomers, diastereomers and structural isomers ;
2. enantiomers, identical, structural isomers and diastereomers ;
3. enantiomers, identical, diastereomers and structural isomers ;
4. identical, identical, diastereomers and structural isomers.
[2011]
Explanation
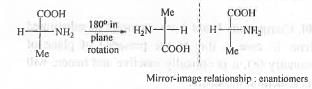
Hence, the pairs in 1 are enantiomers.
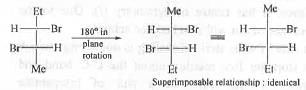
Hence, the pairs in 2 are identical.
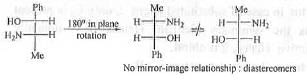
Hence, the pairs in 3 are diastereomers.
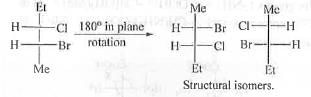
Hence, the pairs in 4 are structural isomers.
Hence, the compounds in 1-4 are enantiomers, identical, diastereomers and structural isomers.
Hence, the correct answer is : C.
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
Try yourself:The Cahn-Ingold-Prelog (CIP) priorities of the groups and the absolute configuration (R/S) of the following compound are
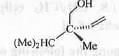
1. -CH2OH > -CH(Me)2 > -CH=CH2 > -CH3 and S ;
2. -CH2OH > -CH=CH2 > -CH(Me)2 > -CH3 and S ;
3. -CH2OH > -CH=CH2 > -CH(Me)2 > -CH3 and R ;
4. -CH2OH > -CH(Me)2 > -CH=CH2 > -CH3 and R.
[2011]
Explanation
Cahn-Ingold-Prelog (CIP) rule is the atomic no. rule. The given groups can represented as follows :
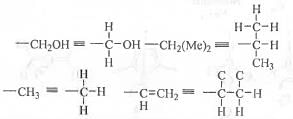
The priority of the various atoms in accordance to atomic no. : -O > - C > -H.
Hence, the priority order of these groups are as follows : -CH2OH > -CH=CH2 > -CH(Me)2 > -CH3
The absolute configuration of the given compound is as follows:
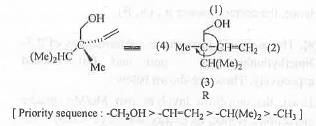
But, the 4-th group is at the horizontal plane. Hence, applying the Very Good Rule, the absolute configuration of the compound will be : S.
Hence, the correct answer is : B.
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
Try yourself:The absolute configurations at the two chiral centres in D-Ribose are
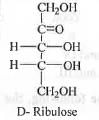
[2010]
Explanation
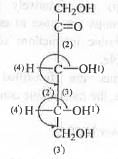
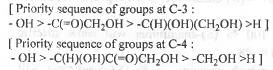
At the C-3 centre : The rotation from 1 → 2 → 3 occurs in anti-clockwise direction. But, as the 4-th group is at the horizontal plane, here Very Good Rule is to be applied. Hence, the final configuration at the C-3 is 3R.
At the C-4 centre : The rotation from 1 → 2 → 3 occurs in anti-clockwise direction. But, as the 4-th group is at the horizontal plane, here Very Good Rule is to be applied. Hence, the final configuration at the C-4 is 4R.
Hence, the absolute configurations at the two chiral centres in D-Ribose are : (3R, 4R).
Hence, the correct answer is : A.
Report a problem
Question for Past Year Question: Stereochemistry Of Organic Molecules
Try yourself:The maximum no. of stereoisomers possible for 4-Phenylbut-3-en-2-ol is
[2009]
Explanation
The compound 4-Phenylbut-3-en-2-ol contains one double bond. An alkcne of type Cab = Ca/b/ will show geometrical isomerism if and only if a ≠ b and a/ ≠ b/ but a/ a/ and b/b/ may be same or, different.
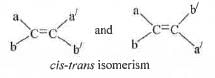
Hence, it can exist as a pair of geometrical isomers. The structure of the isomers are as follows :

Both the geometrical isomers contains one chiral carbon. Hence, each of them will have 21 = 2 optical isomers. Hence, the total no. of stereoisomers of the given compound are 4.
Hence, the correct answer is : D.
Report a problem