Pericyclic Reactions in Details (Part - 2) | Organic Chemistry PDF Download
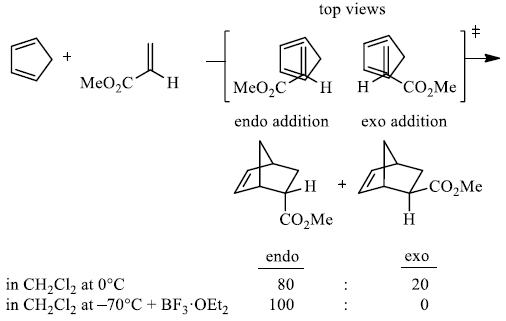
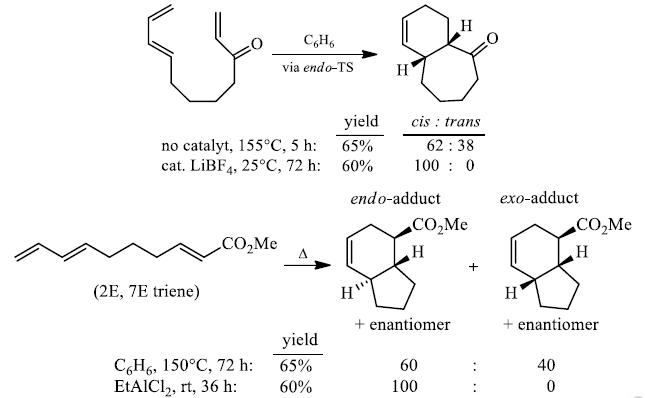
Endo-principle. The dienophile can undergo reaction via two different orientations with respect to the plane of the diene (endo or exo). Maximum overlap of the n-orbitals of the diene and dienophile favors formation of the endo-adduct (secondary orbital overlap between the dienophile's activating substituent and the diene).
exo-Transition state (–CO2Me away from π-system of diene)
endo-Transition state (–CO2Me toward π-systenm diene)
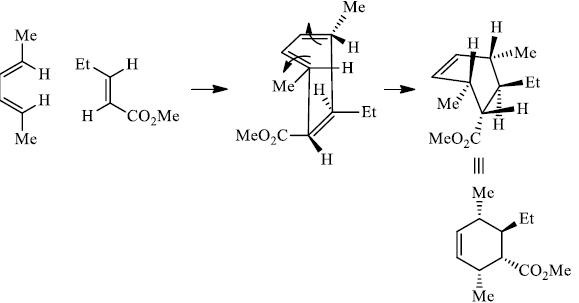
Note that the endo-product has the electron-withdrawing group cis to the (E, E)- substituents of the diene. Only one mode of stacking of the reagents is shown, with the diene above the dienophile. The other possibility is with the diene below the dienophile, leading to the enantiomer.
In general, endo-selectivity is determined by a balance of electronic and steric effects. The endoselectivity may be improved by using Lewis acid catalysis to lower the temperature required for cycloaddition, as shown below.
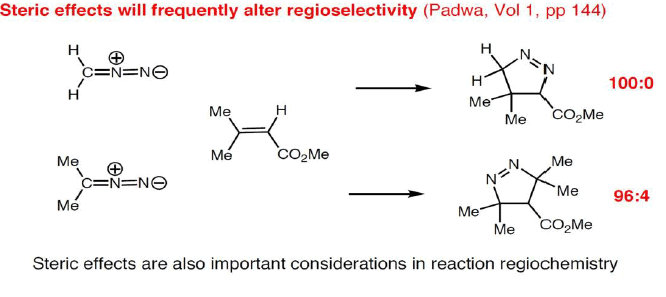
The diene will approach the dienophile from the face opposite a bulky substituent, and a dienophile will approach the less hindered face of the diene.
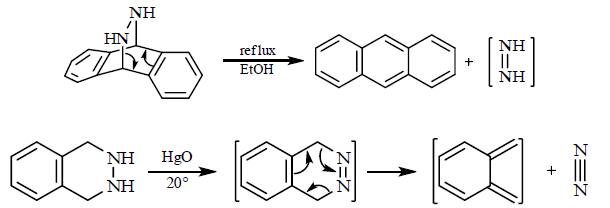
6. Retro Diels-Alder reactions: Diels-Alder reactions are of course, reversible and the pathway followed for the reverse reaction can sometimes be as telling as the pathway for the forward reaction. The direction in which any pericyclic reaction takes place is determined by thermodynamics, with cycloadditions, like the diels-Alder reaction, usually taking place to form a ring because two π-bonds on the left are replaced by two σ-bonds on the right. A Diels-Alder reaction can be made to take place in reverse when the products do not react with each other rapidly.
7. Intramolecular Cycloadditions
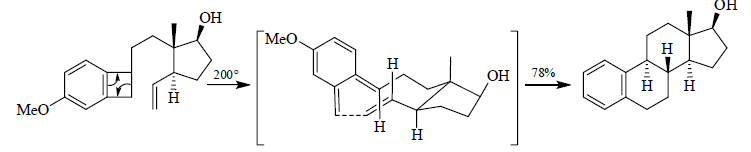
As with intermolecular D-A reactions, the diastereoselectivity of intrainolecular cycloadditions can be improved by the addition of Lewis acids.
8. 1, 3-Dipolar Cycloadditions
1, 3-Dipoles react with alkenes or alkynes, or with heteroatom-containing double and triple bond like carbonyl groups or nitriles, to form heterocyclic rings. The kind of dipoles that feature in 1, 3-dipolar cycloadditions are isoelectronic with an allyl anion, having a conjugated system of three p-orbital on three atoms, X, Y and Z, and four electrons in the conjugated system. The range of possible structures is large; X, Y and Z are commonly almost any combination of C, N, O and S, with a double bond. Likewise, the dipolarophiles, analogous to dienophiles, have a double or triple bond between any pair A and B of the same common elements.
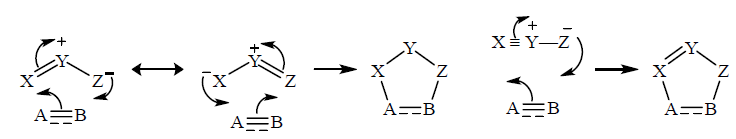
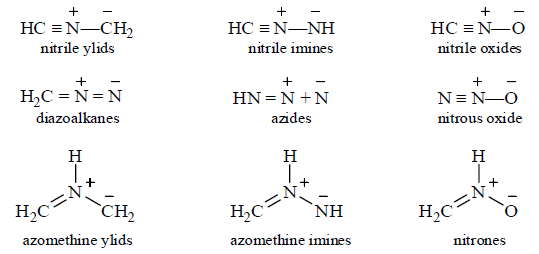
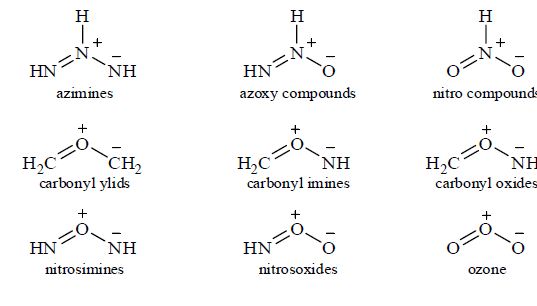
Below are shown the core structures of the most important 1, 3-dipoles, and what they are all called. As with dienes, they can have electron-donating or withdrawing substituents attached at any of the atoms with a hydrogen atom in the core structure, and these modify the reactivity and selectivity that the dipoles show for different dipolarophiles. Some of the dipoles are stable compounds like ozone and diazomethane, or, suitably substituted, like azides, nutrones, and nitrile oxides. Other like the ylids mines, and carbonyl oxides are reactive intermediates that have to be made in situ.
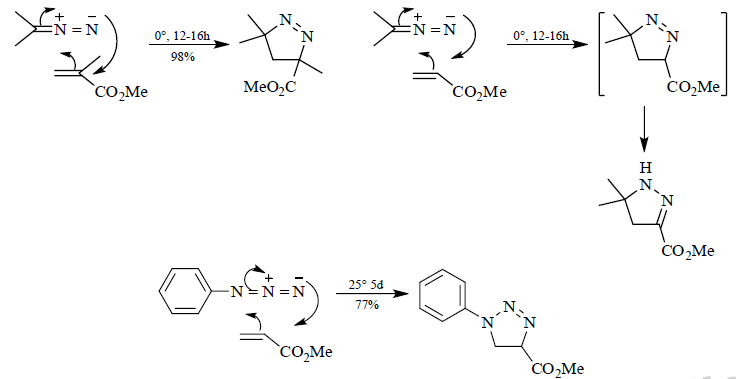
Azide Cycloadditions
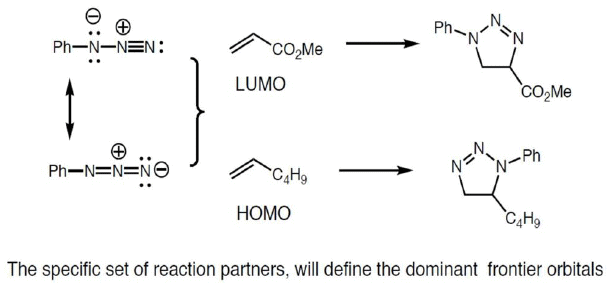
Nitrile Ylide Cycloadditions
Diazoalkane Cycloadditions
Nitrile Oxide Cycloadditions
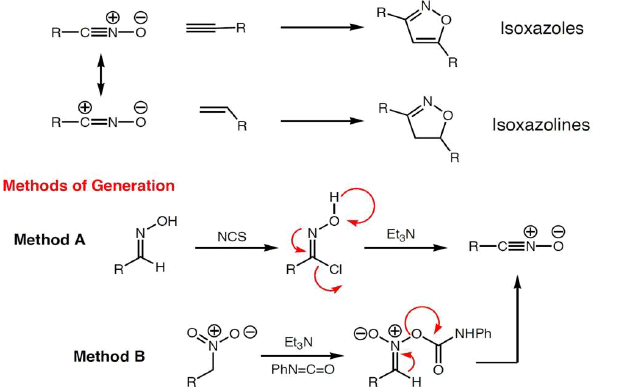
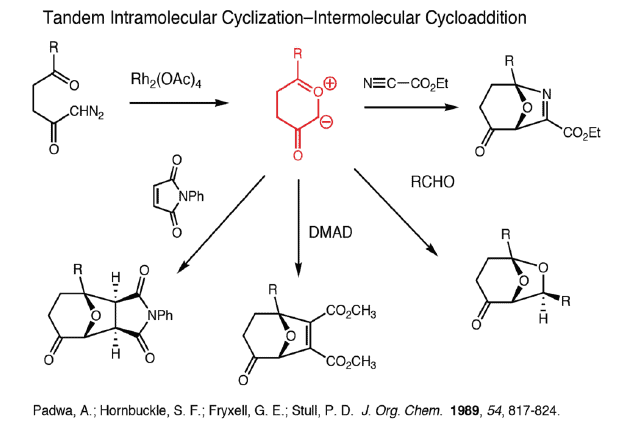
Carbonyl Ylide Cycloadditions
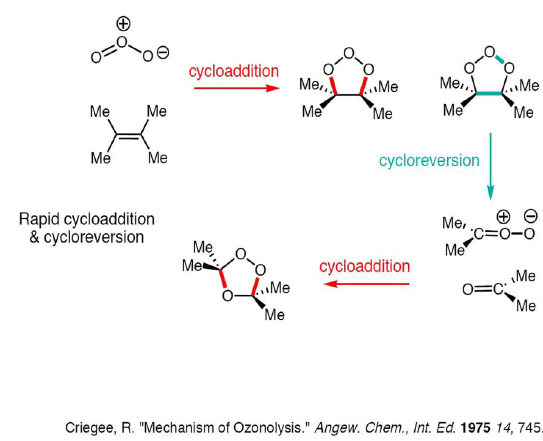
Ozonolysis: The Prototypical Case
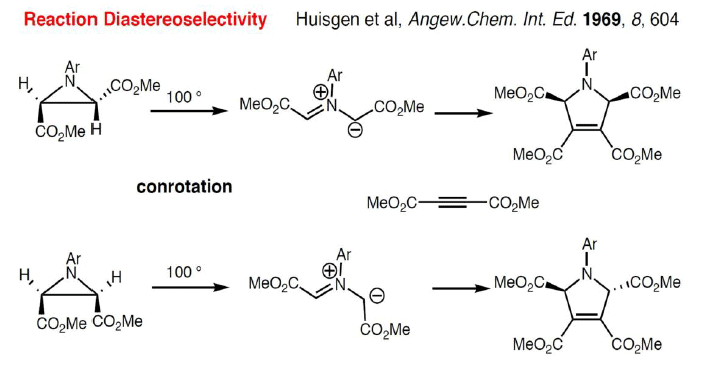
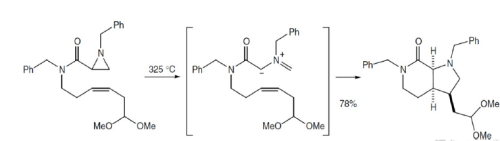
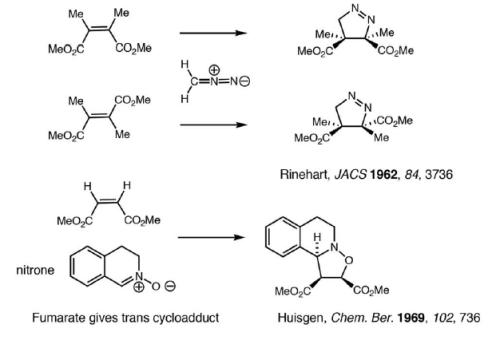
Reaction Stereospecificity with dipolarophile
Some good examples:
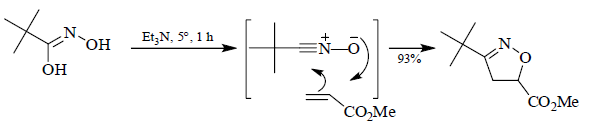
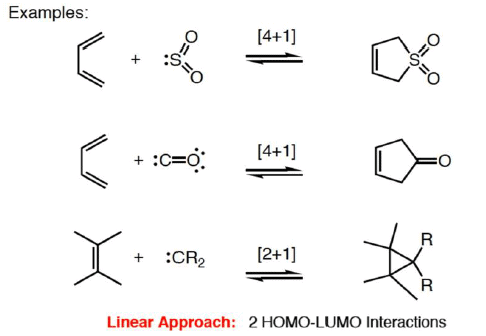
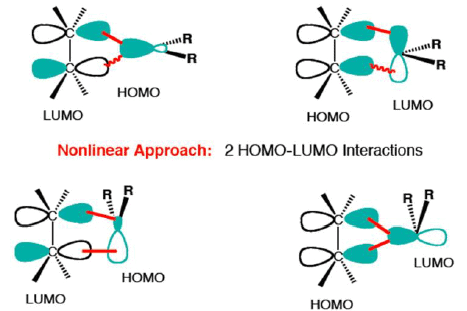
3. CHELETROPIC REACTION
- A special group of cycloaddition/cycloreversion reactions.
- Two bonds are formed or broken at a single atom.
- The nomenclature for cheletropic reactions is the same as for cycloadditions.
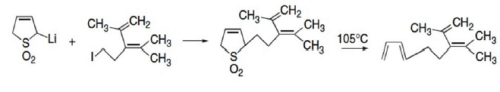
Sulfolene dioxide
Sulfolene dioxide is subject to α-lithiation and alkylation, and this reaction has been used to introduce the ring into more complex molecules.
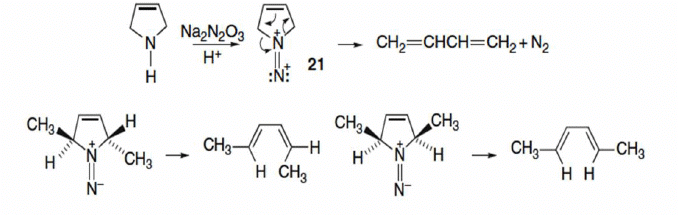
Diazene
Concerted cheletropic elimination is the reaction of 3-pyrroline with N-nitrohydroxylamine gives rise to the diazene 21, which then undergoes elimination of nitrogen.
CO elimination
Elimination of CO from bicyclo[2.2.1]heptadien-7-ones is very facile – almost spontaneous!
|
44 videos|102 docs|52 tests
|
FAQs on Pericyclic Reactions in Details (Part - 2) - Organic Chemistry
| 1. What are pericyclic reactions? |  |
| 2. What are the different types of pericyclic reactions? |  |
| 3. How do pericyclic reactions differ from other types of reactions? |  |
| 4. What is the significance of pericyclic reactions in organic chemistry? |  |
| 5. Can you provide an example of a pericyclic reaction? |  |

















