Periodic Classification of Elements | General Awareness for SSC CGL PDF Download
Evolution of the Periodic Table
- With the discovery of a large number of elements and their compounds, studying these individually became difficult.
- It was necessary to classify the elements into groups to make their study more systematic and easier.
- Various attempts were made, including Prout's unitary theory, Dobereiner's triads law, Newland's law of octaves, and Lother Meyer's curve.
- However, these methods failed to arrange all the known elements.
- The first significant attempt in this direction was made by Mendeleev, who arranged the elements in order of increasing atomic masses.
Mendeleev’s Periodic Table (1869)
Mendeleev's periodic table was based on his periodic law, which stated that "the physical and chemical properties of elements are a periodic function of their atomic masses." His table contained seven periods and eight groups, but the 0 group (inert gases) was absent. Mendeleev left gaps for elements not known at that time, such as Eka-boron, Eka-aluminium, and Eka-silicon, which were later discovered as scandium, gallium, and germanium, respectively. The table lacked a fixed position for hydrogen, had no space for isotopes, and did not arrange atomic masses regularly, necessitating its modification.
Modern Periodic Law
In 1913, Moseley modified Mendeleev’s periodic law and proposed the modern periodic law, stating that "the physical and chemical properties of the elements are a periodic function of their atomic numbers." Moseley discovered that atomic number is a more fundamental property than atomic mass.
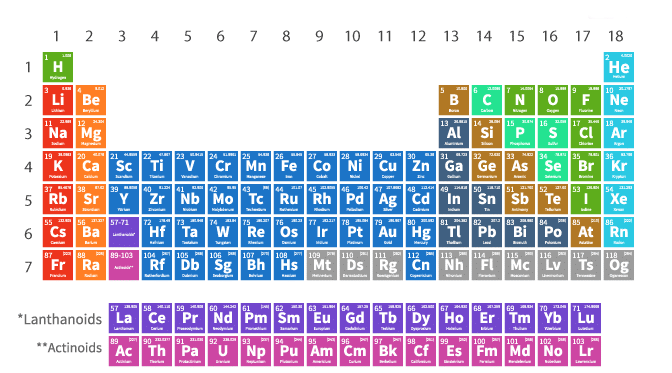
Long Form of Periodic Table
- The long form of the periodic table, or Bohr's table, is based on the Bohr-Burry concept of electronic configuration.
- It contains seven periods (horizontal rows) and eighteen groups (vertical columns).
- The period number corresponds to the number of outermost shells of an element.
- The first element of each period (except the first period) is an alkali metal, and the last element is an inert gas.
- Lanthanoids and actinoids, belonging to the sixth and seventh periods, are placed in two separate rows at the bottom of the table.
- All members of a group have similar outer shell electronic configurations.
- Groups 1, 2, 13, 14, 15, 16, 17, and 18 are known as normal or representative elements, while groups 3 to 12 are known as transition elements.
- The table, based on the electronic configuration of elements, includes 118 elements and follows the Aufbau principle.
- Elements with atomic numbers 3, 11, 19, 37, 55, and 87, having similar properties, belong to the same group.
Characteristics of Periods:
- The number of valence electrons in elements increases from 1 to 8 as you move from left to right in a period.
- Elements in a period have consecutive atomic numbers.
- The valency of elements increases from 1 to 4 and then decreases to 0 as you move from left to right in a period with respect to hydrogen.
Characteristics of Groups:
- All elements in a group have the same number of valence electrons, leading to similar chemical properties.
Block Elements:
Elements can be classified into four blocks:
- s-Block elements: Groups 1 and 2, including hydrogen, alkali metals (Li, Na, K, Rb, Cs, Fr), and alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra), with general configuration ns1 − 2. These elements are soft metals, electropositive, and form basic oxides.
- p-Block elements: Groups 13 to 18, with general configuration ns2np1 − 6. This block includes metals, non-metals, and metalloids. Heavier elements show an inert pair effect, making their lower valency more stable.
- d-Block elements: Groups 3 to 12, with general configuration (n − 1)d1 − 10ns1 − 2. These are transition elements (except group 12), containing unpaired electrons and showing variable valency due to the small energy difference between the outer and penultimate shells. These elements are generally colored and used as catalysts.
- f-Block elements: Positioned below the main periodic table, comprising lanthanides and actinides with general configuration (n − 2)f1−14(n − 1)d0 − 1ns2. These inner-transition elements belong to group IIIB (3).
New Super Heavy Element 117Researchers at the GSI Helmholtz Center for Heavy Ion Research in Darmstadt, Germany, have created and observed several atoms of element 117, temporarily named ununseptium. In the periodic table, ununseptium is located in group 17. While previous group 17 members are halogens, ununseptium is likely to have significantly different properties, although key properties such as melting point, boiling point, and ionization potential are expected to follow periodic trends.
Periodic Properties
 Periodic properties are characteristics that repeat at regular intervals in the periodic table, displaying a consistent pattern along groups and periods. Some important periodic properties include:
Periodic properties are characteristics that repeat at regular intervals in the periodic table, displaying a consistent pattern along groups and periods. Some important periodic properties include:
Ionisation Enthalpy: The minimum energy required to remove an electron from an isolated gaseous atom of an element, forming a positive ion. Ionisation enthalpy increases along a period but decreases down a group. Boron and oxygen have lower ionisation energies than beryllium and nitrogen, respectively, due to their stable electronic configurations.
Electron Gain Enthalpy: The energy released when an extra electron is added to a neutral gaseous atom of an element. Electron gain enthalpy increases along a period and decreases down a group, with some exceptions.
Electronegativity: The ability of an atom to attract a shared pair of electrons towards itself. Electronegativity increases along a period and decreases down a group.
Metallic Character: The tendency of an element to form a cation by losing electrons. Metallic character decreases along a period and increases down a group.
Oxidising and Reducing Character: Reducing character decreases along a period and increases down a group, while oxidising character increases along a period and decreases down a group.
Valency: Valency with respect to hydrogen increases from 1 to 7 across a period. With respect to oxygen, it first increases from 1 to 4 and then decreases to 1 (except for OF2). Valency remains the same within a group.
Basic Nature of Oxides: The basic nature of oxides decreases along a period while the acidic nature increases. Conversely, down a group, the basic nature of oxides increases while the acidic nature decreases.
Hydrogen

Hydrogen is a non-metal that becomes metallic under very high pressure. It constitutes about 10% of the weight of living organisms and is the most abundant element in the universe, making up 70% of the universe's total mass. In its combined state, hydrogen is the third most abundant element in the Earth's crust. It is a fundamental component of the sun and stars.
Various Forms of Hydrogen
- Nascent Hydrogen: This form of hydrogen appears suddenly during a chemical reaction and is more reactive than molecular hydrogen.
- Atomic Hydrogen: Produced by the decomposition of molecular hydrogen.
- Ortho Hydrogen: In this form, the nuclei of the hydrogen atoms in the molecule revolve in the same direction.
- Para Hydrogen: In this form, the nuclei of the hydrogen atoms in the molecule revolve in opposite directions.
Isotopes of Hydrogen
- Protium (1H1): This isotope has an atomic number and mass number of 1.
- Deuterium (1H2): Known as heavy hydrogen, it has an atomic number of 1 and a mass number of 2. Discovered by Urey, Brickwedde, and Murphy in 1931, it is used to explain organic reaction mechanisms and as bombarding particles in nuclear reactions.
- Tritium (1H3): A rare and radioactive isotope with an atomic number of 1 and a mass number of 3. It is a beta emitter with a half-life of 12.4 years.
Compounds of Hydrogen
- Water (H2O): Water makes up about 65% to 95% of living beings and exists in three physical states: solid (ice), liquid (water), and gas (water vapor). It is a colorless, mobile, and volatile liquid with unique properties due to extensive hydrogen bonding between water molecules. Drinking water is purified through boiling, chlorination, ozonization, or ultraviolet radiation.
Types of Water:
- Soft Water: Forms lather with soap.
- Hard Water: Does not form lather with soap. Hardness is due to the presence of calcium or magnesium bicarbonates (temporary hardness) or chlorides or sulfates of calcium or magnesium (permanent hardness).
Degree of Hardness
The degree of hardness is defined as the number of parts of CaCO3 or its equivalent in various calcium or magnesium salts present in 106 parts of water by mass.
Heavy Water (D2O):
Discovered by Urey and Washburn in 1932, heavy water is present in a ratio of one part per 6000 parts of ordinary water. It is a colorless, odorless, tasteless liquid with a maximum density of 1.1073 g/mL at 11.6°C. Heavy water has higher physical constants than ordinary water due to the greater nuclear mass of deuterium and stronger hydrogen bonding. Chemically, it reacts more slowly than ordinary water due to the isotopic effect.
Effects of Heavy Water:
- Biochemical reactions are significantly affected.
- Seeds do not germinate in heavy water.
- The rate of fermentation decreases.
- The growth of bio-organisms is retarded.
- Aquatic animals like tadpoles and fish die in heavy water.
Hydrogen Peroxide (H2O2):
Discovered by Thenard, hydrogen peroxide is also known as oxygenated water and is found in trace amounts in the atmosphere, plants, rain, and snow. It decomposes in light and is stored in dark-colored or wax-lined bottles to prevent decomposition.
Uses of Hydrogen Peroxide (30% Perhydrol):
- As a germicide and antiseptic for wounds, ears, and teeth.
- As a preservative for milk and protein.
- As a bleaching agent for wool and other soft materials.
|
477 videos|1380 docs|312 tests
|
FAQs on Periodic Classification of Elements - General Awareness for SSC CGL
| 1. What are the periodic properties of elements? |  |
| 2. What is the significance of hydrogen in the periodic table? |  |
| 3. How is the periodic table classified in SSC CGL exams? |  |
| 4. What are some common periodic trends tested in SSC CGL exams? |  |
| 5. How does the evolution of the periodic table contribute to our understanding of chemical elements? |  |
















