Class 7 Science Chapter 2 Previous Year Questions - Acids, Bases and Salts
Q.1. Write the equation for the dissociation of hydrochloric acid (HCl) in water.
Ans. HCl + H2O → H3O+ + Cl-
or it can also be written as
HCl  H+(aq) + Cl-(aq)
H+(aq) + Cl-(aq)
Q.2. Which one of these has a higher concentration of H+ ions?
1M HCl or 1M CH3COOH
Ans. 1M HCl will have a higher concentration of H+ ions because HCl being strong acid undergoes complete dissociation.
Q.3. While diluting an acid why is it recommended that the acid should be added to water and not water to the acid?
Or
Why should water be never added dropwise to concentrated sulphuric acid?
Ans.
While diluting an acid, water should not be added to a concentrated acid because adding water to acid is a highly exothermic process and the heat generated may cause the mixture to splash out.
Q.4. How is the concentration of hydroxide ions (OH-) affected when excess base is dissolved in a solution of sodium hydroxide?
Ans. The concentration of hydroxide ions (OH-) is increased when the excess base is dissolved in a solution of sodium hydroxide.
Q.5. Do basic solutions also have H+ (aq) ions? If yes, then why are these basic?
Ans. Basic solutions also have H+ (aq) ions. A solution of an acid or a base always contains both H+ (aq) ions as well as OH- (aq) ions. It shows the basic character if it has more OH- (aq) ions and acidic character if it has more H+ (aq) ions.
Q.6. Choose strong acid and a strong base from the following: CH3COOH, NH4OH, KOH, HCl
Ans. A strong acid is HCl and strong base is KOH.
Q.7. Which is more acidic a solution with pH = 6.0 or a solution with pH = 2.0?
Ans. A solution with pH = 2.0 is more acidic.
Q.8. Which is more basic, a solution with pH = 9.0 or a solution with pH = 13.0?
Ans. A solution with pH = 13.0 is more basic.
Q.9. How would you show that lemon and tomato contain acids?
Ans. Both, lemon juice and tomato juice turn blue litmus red. It shows that both of them contain acids.
Q.10. What is the action of the solution of sodium carbonate towards litmus?
Ans. Solution of sodium carbonate will turn the color of red litmus into blue indicating that it is alkaline in nature.
Q.11. Dry ammonia gas has no action on litmus paper but a solution of ammonia in water turns red litmus paper blue. Why is it so?
Ans. Ammonia in water forms ammonium hydroxide. These hydroxide ions turn red litmus blue.
Q.12. What is the action on litmus of:
(a) Dry ammonia gas?
(b) The solution of ammonia gas in the water?
Ans.
(a) Dry ammonia gas has no action on litmus.
(b) Solution of ammonia gas in water turns red litmus blue.
Q.13. Why should curd and sour substances not be kept in brass and copper vessels?
Ans. Curd and sour substance contain acids which react with brass and copper and forms toxic substances whose consumption is bad for health.
Q.14. Why do HCl, HNO3, etc. show acidic character in aqueous solutions while solutions of compounds like C2H5OH and glucose do not show acidic character?
Ans. A substance will show an acidic character if it gives H+ ions when dissolved in water. Among these substances, HCl and HNO3 provide H+ ions whereas C2H5OH and glucose do not give H+ ions so they do not show acidic character.
Q.15. Given two unlabelled bottles, one containing dilute acid and the other water. How would you decide to label them?
Ans. Acid and water can be identified by testing with litmus. Water will not change the colour of red or blue litmus whereas acid will change blue litmus into the red.
Q.16. Why does distilled water not conduct electricity, whereas rainwater does?
Ans. The electric current is carried by ions in solutions. Distilled water has no ions whereas rainwater is slightly acidic and contains ions so rainwater conducts electricity.
Q.17. What happens when carbon dioxide gas is passed through sodium hydroxide solution?
Ans. When carbon dioxide gas is passed through sodium hydroxide solution, sodium carbonate is formed.
2NaOH + CO2 → Na2CO3 + H2O
Q.18. Name the sodium compound which is used for softening hard water.
Ans: The sodium compound used for softening hard water is sodium carbonate (Na2CO3.10H2O) also called sodium carbonate decahydrate
Q.19. What are the chemical name and formula of baking soda?
Ans. The chemical name of baking soda is sodium hydrogen carbonate and its formula is NaHCO3.
Q.20. A compound 'X' is an important ingredient of an antacid. It is also used in fire extinguishers. Identify 'X'.
Ans. Compound 'X' is sodium hydrogen carbonate (NaHCO3).
Q.21. Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
Or
Fresh milk has a pH of 6. When it changes into curd (yogurt) will its pH value increase or decrease? Why?
Ans. The pH will decrease from 6 because it becomes more acidic when milk is converted into curd and more acidic solutions have lower pH value.
 Fig: pH scale
Fig: pH scale
Q.22. What happens when crystals of washing soda are left open in dry air? What is this change named as? Name two industries based on the use of washing soda.
Ans. When crystals of washing soda are left open in dry air, they lose nine molecules of water of crystallisation and become white powder.
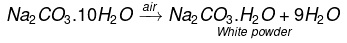
This change is called efflorescence.
Two industries based on the use of washing soda are:
(i) manufacture of glass
(ii) paper and textile industries.
Q.23. What will happen if the solution of sodium hydrogen carbonate is heated? Give the equation of the reaction involved.
Or
(i) Name the products formed when sodium hydrogen carbonate is heated.
(ii) Write the chemical equation for the reaction involved in the above.
Ans. When the solution of sodium hydrogen carbonate is heated, it decomposes to form sodium carbonate with the evolution of carbon dioxide gas. 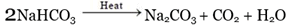
Q.24. How is Plaster of Paris chemically different from gypsum? How may they be interconverted? Write one use of Plaster of Paris.
Or
How is Plaster of Paris obtained? What reaction is involved in the setting of a paste of Plaster of Paris?
Or
State the chemical difference between Plaster of Paris and gypsum. Describe their either-way interconversions.  Fig: Plaster of Paris
Fig: Plaster of Paris
Ans. Plaster of Paris is chemically different from gypsum in terms of water of crystallisation. Gypsum has 2 moles of water per mole of CaSO4, (CaSO4.1/2H2O). It can also be written as if one mole of water of crystallisation is present for two moles of CaSO4, (2CaSO4. H2O). Gypsum on heating at 373 K gets converted into Plaster of Paris.
When Plaster of Paris is mixed with water, it gets converted into gypsum.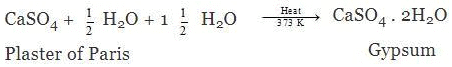
The plaster of Paris is used for making statues and for setting fractured bones.
Q.25. Name three compounds of calcium that are used in day-to-day life and write one important use of each of them.
Ans. The three compounds of calcium and their uses are :
(i) Slaked lime [Calcium hydroxide, Ca(OH)2] used for the manufacture of bleaching powder.
(ii) Bleaching powder [Calcium oxychloride, CaOCl2] used as a bleaching agent in the laundry.
(iii) Plaster of Paris [Calcium sulfate hemihydrate, CaSO4. 1/2H2O] used to plaster the fractured bones.
PREVIOUS YEARS' BOARD QUESTIONS
Q.26. A chemical compound having the smell of chlorine is used to remove the yellowness of white clothes in the laundries. Name the compound and write the chemical equation involved in its preparation.
Ans. The compound is bleaching powder (CaOCl2). It removes yellowness from clothes due to its bleaching action. For details, consult the text part.
Q.27. Explain giving reasons:
(i) Tartaric acid is a component of baking powder used in making cakes.
(ii) Gypsum, CaSO4.2H2O is used in the manufacture of cement.
Ans.
(i) Role of tartaric acid in baking powder (mixture of tartaric acid and sodium hydrogen carbonate) is to neutralise sodium carbonate formed upon heating sodium hydrogen carbonate.
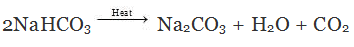
Sodium hydrogen carbonate Sodium carbonate
(ii) The role of gypsum (CaSO4.2H2O) in the manufacture of cement is to slow down the process of setting of cement.
Q.28. How is chloride of lime chemically different from calcium chloride? Why does chloride of lime gradually lose its chlorine when kept exposed to air?
Ans. Chloride of lime is calcium oxychloride [(Ca(OCl)Cl] also known as bleaching powder. Calcium chloride is CaCl2. Bleaching powder gives identific smell of chlorine when exposed t air because it reacts with CO2 and chlorine gas is evolved. 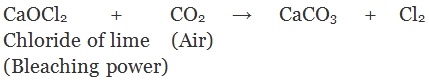
Q.29. What is the chemical name of washing soda? Name three raw materials used in making washing soda by Solvay process.
Ans. Chemical name: Sodium carbonate decahydrate (Na2CO3.10 H2O).
Raw materials: Brine, limestone, ammonia.
Q.30. State the chemical property in each case on which the following uses of baking soda are based
(i) as an antacid.
(ii) as a constituent of baking powder.
Ans.
(i) It is weakly alkaline in nature and neutralizes acid (HCl) formed in the stomach.
NaHCO3 + HCl → NaCl + H2O + CO2
(ii) It evolves CO2 in the form of bubbles when cake is made by baking. As a result, the cake becomes porous as well as fluffy.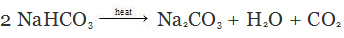
Q.31.
(a) Name the raw materials used in the manufacture of sodium carbonate by Solvay process.
(b) How is sodium hydrogen carbonate formed during Solvay process separated from a mixture of NH4Cl and NaHCO3 ?
(c) How is sodium carbonate obtained from sodium hydrogen carbonate?
Ans.
(a) The raw materials used are: NaCl, limestone or CaCO3 and NH3.
(b) Sodium hydrogen carbonate (NaHCO3) is sparingly soluble or less soluble in water and gets separated as a precipitate while NH4Cl remains in solution. The precipitate is removed by filtration.
(c) Sodium hydrogen carbonate is converted to sodium carbonate upon heating.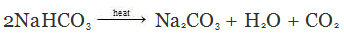
For further details, consult the text part.
Q.32.
(a) What is the action of red litmus on (i) dry ammonia gas (ii) solution of ammonia gas in water
(b) State the observations you would make on adding ammonium hydroxide to an aqueous solution of
(i) ferrous sulfate (ii) aluminum chloride.
Ans.
(a)
(i) Red litmus has no action on dry ammonia gas because it does not release any hydroxyl ions (OH-)
(ii) When passed through water, ammonia (NH3) is converted to ammonium hydroxide (NH4OH). It dissociates to give hydroxyl ions (OH-) and the solution is basic in nature. Red litmus acquires a blue color.
(b)
(i) A green precipitate of ferrous hydroxide will be formed by double decomposition reaction.
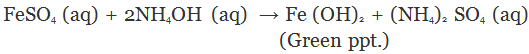
(ii) A white precipitate of aluminium hydroxide will be formed by double decomposition reaction.
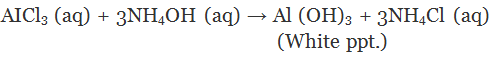
Q.33. Name the gas that evolved when dilute HCl reacts with sodium hydrogen carbonate. How is it recognised?
Ans. The gas that evolved is carbon dioxide (CO2). When the gas is bubbled through lime water, it becomes milky due to the formation of CaCO3.
Q.34.
(a) Name the two chief chemicals used for making a soda acid fire extinguisher.
(b) How does the soda-acid fire extinguisher help to extinguish the fire?
Ans.
(a) The two chief chemicals are sodium hydrogen carbonate (NaHCO3) and sulphuric acid (H2SO4)
(b) For the details of the operation, consult the text part.
Q.35. How will you test for the gas which is liberated when hydrochloric acid reacts with an active metal?
Ans. Hydrogen gas is evolved when hydrochloric acid reacts with an active metal such as sodium, potassium,
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
calcium or magnesium. In order to test the gas, bring either a burning match stick or candle near the gas. The gas will immediately catch fire and burns with a ‘pop’ sound.
Q.36. An aqueous solution has a pH value of 7.0. Is this solution acidic, basic, or neutral?
Ans. The solution with a pH value of 7.0 is neutral in nature
Q.37. Out of calcium compounds calcium carbonate, quick lime, and slaked lime, which one can be used for removing moisture from ammonia gas and why?
Ans. Quick lime (CaO) can be used to remove moisture from ammonia gas because of its hygroscopic nature. Therefore, it can act as the best dehydrating agent for ammonia.
|
12 videos|50 docs|17 tests
|
FAQs on Class 7 Science Chapter 2 Previous Year Questions - Acids, Bases and Salts
| 1. What is the pH of an acidic solution? |  |
| 2. What are some common household acids and bases? |  |
| 3. How do acids and bases react with metals? |  |
| 4. What is the difference between a strong acid and a weak acid? |  |
| 5. How do you determine if a substance is an acid or a base? |  |

















