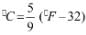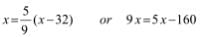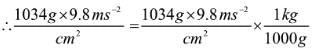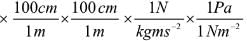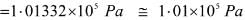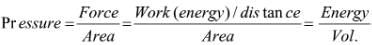Properties Of Matter & Their Measurement | Additional Study Material for JEE PDF Download
Property of Matter
- Physical Property: The property which can be measured without changing the chemical composition of the substance is known as physical property.
Example: mass, volume, density, refractive index, boiling point, melting point, etc. - Chemical Property: The property which can be evaluated at the cost of matter itself is known as chemical property.
Example: oxygen gas can be verified by burning of flame in presence of oxygen gas.
Units for Measurement
All physical quantities have to be measured. The value of a physical quantity is expressed as the product of the numerical value and the unit in which it is expressed.
Fundamental Units
Fundamental units are those units which can neither be derived from one another nor they can be further resolved into any other units.
- Metric System was based on the decimal system.
- The International System of Units (SI)
The International System of Units (in French Le Systeme International d’Unites– abbreviated as SI) was established by the 11th General Conference on Weights and Measures (CGPM from Conference Generale des Poids at Measures).
The SI system has seven base units.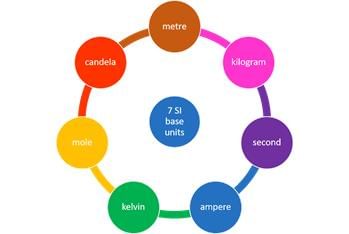 Seven Basic S.I. Units
Seven Basic S.I. Units
The Seven Fundamental Units of measurement in S.I. system.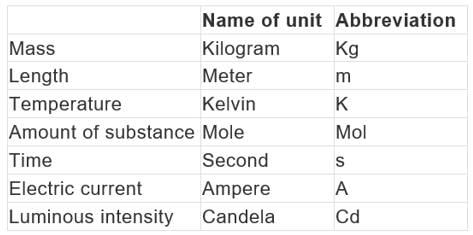
Definitions of S.I. Base Units
- Unit of length: Metre
The meter is the length of the path travelled by light in vacuum during a time interval of 1/299 792 458 of a second. Unit of mass kilogram - The kilogram is the unit of mass; it is equal to the mass of the international prototype of the kilogram.
- Unit of time second The second is the duration of 9 192 631 770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium-133 atom.
- Unit of electric current ampere: The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2 × 10–7 newton per meter of length.
- Unit of thermodynamic kelvin: is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water.
- Unit of amount of substance mole: The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12; its symbol is “mol.” 2. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
- Unit of luminous intensity candela: The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 × 1012 hertz and that has a radiant intensity in that direction of 1/683 watt per steradian.
Derived units: Some quantities are expressed as a function of more than one fundamental unit known as derived units.
Example: velocity, acceleration, work, energy, area, density, etc.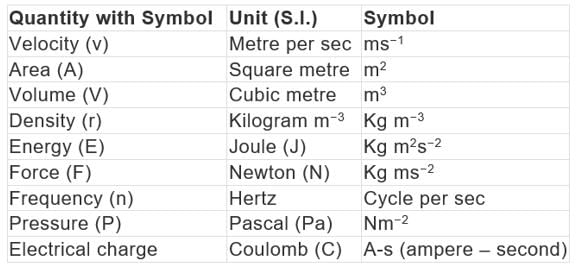
Try Yourself!
1Pa = 1Nm-2
If the mass of air at sea level is 1034 g cm-2, the pressure in pascal is
|
22 videos|163 docs|17 tests
|
FAQs on Properties Of Matter & Their Measurement - Additional Study Material for JEE
| 1. What are the fundamental units used for measuring properties of matter? |  |
| 2. How are properties of matter measured? |  |
| 3. What are some common properties of matter that can be measured? |  |
| 4. How do units help in measuring properties of matter? |  |
| 5. Why is it important to measure properties of matter accurately? |  |