Past Year Questions: Quality Characteristics of Water | Environmental Engineering - Civil Engineering (CE) PDF Download
Q1: A 100 mg of HNO3 (strong acid) is added to water, bringing the final volume to 1.0 liter. Consider the atomic weights of H, N, and O, as 1 g/mol, 14 g/mol, and 16 g/mol, respectively. The final pH of this water is (Ignore the dissociation of water.) (2022 SET-2)
(a) 2.8
(b) 6.5
(c) 3.8
(d) 8.5
Ans: (a)
Sol: HNO3 → H+ + NO-3
1 mole of HNO3 = 1 mole of H+
No. of mole of HNO3 = 100/(1 + 14 + 16 x 3)
= 1.587m mol
[H+] in 1 lit. of water = 1.587m mol/Lit
= 1.587 x 10-3 mol/Lit
pH = -log[H+]
= log[1.587 x 10-3]
= 2.8
Q2: In a water sample, the concentrations of Ca2+, Mg2+, and HCO-3 are 100 mg/L, 36 mg/L and 122 mg/L, respectively. The atomic masses of various elements are: Ca = 40, Mg = 24, H = 1, C = 12, O = 16.
The total hardness and the temporary hardness in the water sample (in mg/L as CaCO3) will be (2022 SET-1)
(a) 400 and 100, respectively.
(b) 400 and 300, respectively.
(c) 500 and 100, respectively.
(d) 800 and 200, respectively.
Ans: (a)
Sol: Total Hardness,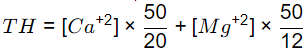
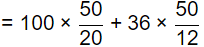
= 400 mg/l as CaCO3
Total alkalinity,
TA = [HCO-3] × (50/61) = 122 × (50/61) = 100mg/l as CaCO3
Temporary hardness (carbonate hardness)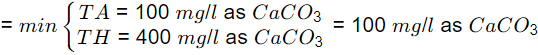
Q1: A water filtration unit is made of uniform-size sand particles of 0.4 mm diameter with a shape factor of 0.84 and specific gravity of 2.55. The depth of the filter bed is 0.70 m and the porosity is 0.35. The filter bed is to be expanded to a porosity of 0.65 by hydraulic backwash. In the terminal settling velocity of sand particles during backwash is 4.5 cm/s, the required backwash velocity is (2021 SET-2)
(a) 5.79 × 10−3 m/s
(b) 6.35 × 10−3 m/s
(c) 0.69 cm/s
(d) 0.75 cm/s
Ans: (b)
Sol: n′ = Porosity of expanded bed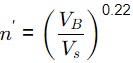
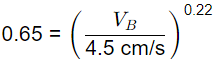
VB = 6.35 x 10-3 m/s
Q2: The internal (di) and external(do) diameters of a Shelby sampler are 48 mm and 52 mm, respectively. The area ratio (Ar) of the sampler (in %,round off to two decimal palces) is _______ (2021 SET-2)
Ans: 17.25 to 17.45
Sol: Outside diameter = 52 mm
Inside diameter = 48 mm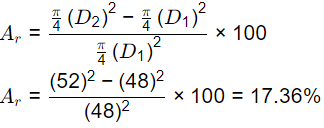
Q3: A water sample is analyzed for coliform organisms by the multiple-tube fermentation method. The results of confirmed test are as follows: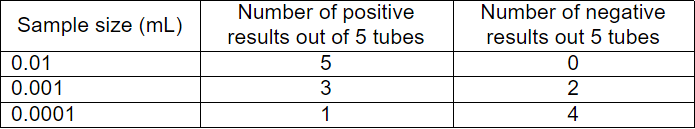
The most probable number (MPN) of coliform organisms for the above results is to be obtained using the following MPN Index.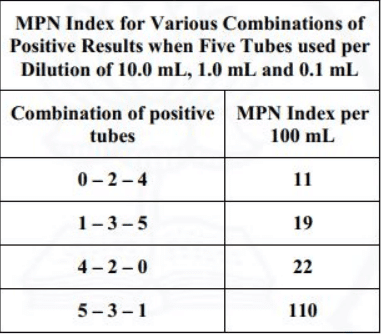
The MPN of coliform organisms per 100 mL is (2021 SET-1)
(a) 1100000
(b) 110000
(c) 1100
(d) 110
Ans: (b)
Sol: The sample size is 0.01 ml, 0.001 ml and 0.0001 ml which is 1000 times lesser than the standard of 10 ml, 1 ml and 0.1 ml.
The positive set is 5-3-1 and w.r.t. the positive combination,
MPN/100 ml will be 110 x 1000 = 110000.
Q4: Which one of the following is correct? (2021 SET-1)
(a) The partially treated effluent from a food processing industry, containing high concentration of biodegradable organics, is being discharged into a flowing river at a point P. If the rate of degradation of the organics is higher than the rate of aeration, then dissolved oxygen of the river water will be lowest at point P.
(b) The most important type of species involved in the degradation of organic matter in the case of activated sludge process based wastewater treatment is chemoheterotrophs
(c) For an effluent sample of a sewage treatment plant, the ratio BOD 5− day ,20ºC upon ultimate BOD is more than 1
(d) A young lake characterized by low nutrient content and low plant productivity is called eutrophic lake
Ans: (b)
Sol: Chemoheterotrophs are the organisms which generate energy with the help of chemical reactions by consuming organic matter during their metabolism. These are the species which help to degrade the organic matter in ASP.
Q1: A sample of water contain an organic compound C8H16O8 at a concentration of 10−3 mol/litre. Given that the atomic weight of C = 12 g/mol, H = 1 g/mol, and O = 16 g/mol, the theoretical oxygen demand of water (in g of O2 per litre, round off to two decimal places), is ___________ (2020 SET-2)
Ans: 0.24 to 0.27
Sol: C8H16O8 of conc 10−3 moles/lt required O2 (in gm/lt)
C8H16O8 + 8O2 → 8CO2 + 8H2O
1 mole of C8H16O8 requires 8 moles of O2 for its decomposition
240 gm = 128 gm
10−3 moles = 8 × 10−3 moles
= 8 × 10−3 x 32
= 0.256 gm/lt
Q2: The ion product of water (pKw) is 14. If a rain water sample has a pH of 5.6, the concentration of OH− in the sample (in 10−9 mol/litre, round off to one decimal place), is ________ (2020 SET-2)
Ans: (3.8 to 4.1)
Sol: pH + pOH = 14
pOH = 14 − 5.6 = 8.4
−log[OH−] = 8.4
[OH−] = 10-8.4moles/lt
= 10-8.4+9 x 10-9 moles/lt
= 3.98 x 10-9 moles/lt
Q3: Alkalinity of water, in equivalent/litre (eq/litre), is given by
{HCO-3} + 2{CO2-3} + {OH−} − {H+}
where, { } represents concentration in mol/litre. For a water sample, the concentration of HCO?3 = 2 × 10−3 mol/litre, CO2-3 = 3.04 × 10−4 mol/litre and the pH of water = 9.0. The atomic weights are : Ca = 40; C = 12; and O = 16. If the concentration of OH− and H+ are NEGLECTED, the alkalinity of the water sample (in mg/litre as CaCO3), is (2020 SET-2)
(a) 130.4
(b) 100
(c) 50
(d) 65.2
Ans: (a)
Sol: Alkalinity of water sample is due to presence of
[HCO-3] and [CO2-3]
Total alkalinity = 1 mole of [HCO-3] + 2 mole of [HCO2−3] in terms of CaCO3
= (2 × 10−3 × 50 + 2 × 3.04 × 10−4 × 50) × 103mg/l
= 130.4 mg/l as CaCO3
Q4: A river has a flow of 1000 million litres per day (MLD), BOD5 of 5 mg/litre and Dissolved Oxygen (DO) level of 8 mg/litre before receiving the wastewater discharge at a location. For the existing environmental conditions, the saturation DO level is 10 mg/litre in the river. Wastewater discharge of 100 MLD with the BOD5 of 200 mg/litre and DO level of 2 mg/litre falls at that location. Assuming complete mixing of wastewater and river water, the immediate DO deficit (in mg/litre, round off to two decimal places), is _________. (2020 SET-1 )
Ans: 2.45 to 2.65
Sol: 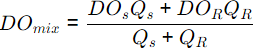
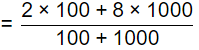
= 7.45 mg/l
DO = DOsat - DOmix
= 10 - 7.45 = 2.545 mg/l
Q5: An amount of 35.67 mg HCI is added to distilled water and the total solution volume is made to one litre. The atomic weights of H and CI are 1 and 35.5, respectively. Neglecting the dissociation of water, the pH of the solution, is (2020 SET-1)
(a) 3.5
(b) 3.01
(c) 2.5
(d) 2.01
Ans: (b)
Sol: HCI → H+ + CI−
1 mole of HCL gives 1 mole H+ ions
36.5 gm of HCl gives 1 gm of H+ ions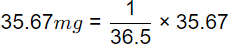
= 0.977 mg of H+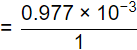
= 9.77 x 10-4 mole of H+
pH = -log10[H+] = -log10[9.77 x 10-4]
= -log109.77 + 4log1010
= 4 - 0.989 = 3.01
Q1: Analysis of a water sample revealed that the sample contains the following species.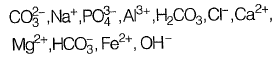
Concentrations of which of the species will be required to compute alkalinity? [2019 : 1 Mark, Set-II]
(a) 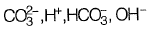
(b) 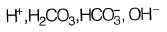
(c) 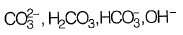
(d) 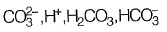
Ans: (a)
Sol: Alkalinity is defined as ability of water to neutralize the acid or hydronium ion
Alkalinity (AT) of water = 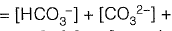
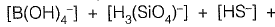 [organic anions]+ [OH-]+ [H+]
[organic anions]+ [OH-]+ [H+]
From given options of ions in problem answer is (a).
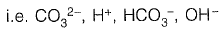
Q1: A water sample analysis data is given below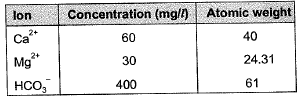
The carbonate hardness (expressed as mg/L of CaCO3, up to one decimal place) for the water sample is ______ [2018 : 2 Marks, Set-I]
Ans: 273.4 mg/l
Sol: Carbonate hardness = min. (Total hardness, alkalinity)
Total hardness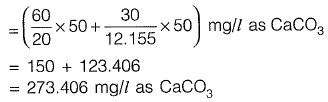
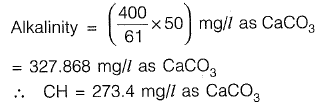
Q2: As per IS 10500:2012, for drinking water in the absence of alternate source of water, the permissible limits for chloride and sulphate, in mg/L, respectively are [2018 : 1 Mark, Set-II]
(a) 250 and 200
(b) 1000 and 400
(c) 200 and 250
(d) 500 and 1000
Ans: (b)
Sol: As per IS-10500 : 2012, Table 2
Permissible limit in absence of alternate source.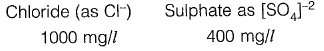
Q1: The analysis of a water sample produces the following results: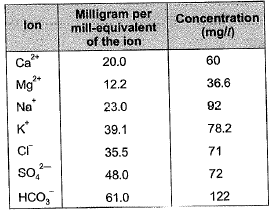
The total hardness (in mg/l as CaCO3) of the water sample is _______. [2017 : 2 Marks, Set-I]
Ans: 300mg/l
Sol: Milligrah per milli-equivalent of the ion
= equivalent weight
∴ Milliequivalent of Ca2+ = 60/20 = 3
and, milliequivalent of Mg+2 36.6/12.2 = 3
Total hardness (due to Mg2+ and Ca2+)
= Milliequivalent of Ca2+ and Mg2+
= 3 + 3 = 6 milliequivalent/l
= (6 x 50) mg/l as CaCO3
= 300 mg/l as CaCO3
Q1: Effluent from an industry ‘A’ has a pH of 4.2. The effluent from another industry 'B' has double the hydroxyl (OH-) ion concentration than the effluent from industry ‘A'. pH of effluent from the industry ‘B’ will be _________ [2016 : 2 Marks, Set-I]
Ans: 4.5
Sol:
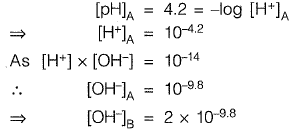
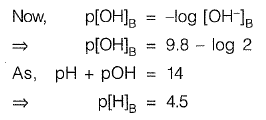
Q2: A sample of water has been analyzed for common ions and results are presented in the form of a bar diagram as shown
The non-carbonate hardness (expressed in mg/l as CaCO3) of the sample is [2016 : 2 Marks, Set-Il]
(a) 40
(b) 165
(c) 195
(d) 205
Ans: (a)
Sol: Hardness is due to multivalent metallic cations,
i.e. Ca2+ and Mg2+
Total hardness (mg/l as CaCO3)
= (Total meq/l) x (eq. weight of CaCO3 in mg)
= (4.1) x 50 mg/l as CaCO3
= 205 mg/l as CaCO3
Alkalinity is due to the presence of  in this case
in this case
Alkalinity (mg/l as CaCO3)
= 3.3 x 50 mg/l as CaCO3
= 165 mg/l as CaCO3 Now, non-carbonate hardness
= Total Hardness in excess of alkalinity
= 205 - 165
= 40 mg/l as CaCO3
Q1: A groundwater sample was found to contain 500 mg/L total dissolved solids (TDS). TDS (in %) present in the sample i s _______ . [2015 : 1 Mark, Set-II]
Ans: 0.05%
Sol: 1 kg = 1 litre
TDS (in %) in the sample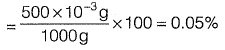
Q1: For a sample of water with the ionic composition shown in the figure below, the carbonate and noncarbonate hardness concentrations (in mg/I as CaCO3), respectively are: [2014 : 2 Marks, Set-I]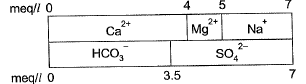
(a) 200 and 50
(b) 175 and 75
(c) 75 and 175
(d) 50 and 200
Ans: (b)
Sol: Total hardness
= 5 meq/l (due to Ca2+ and Mg2+)
= (5 x 50) mg/l as CaCO3
= 250 mg/l as CaCO3
Alkalinity (due to HCO3-)
= 3.5 Meq/l
= (3.5x50) mg/l as CaCO3
= 175 mg/l as CaCO3
Carbonate hardness
= Min. (tota hardness, Alkalinity)
= Minimum (250,175)
= 175 mg/l as CaCO3
Non-carbonate hardness
= Total hardness - Carbonate hardness
= 250 - 175
= 75 mg/l as CaCO3
Q2: Some of the nontoxic metals normally found in natural water are
(a) arsenic, lead and mercury [2014 : 1 Mark, Set-I]
(b) calcium, sodium and silver
(c) cadmium, chromium and copper
(d) iron, manganese and magnesium
Ans: (d)
Sol: Although manganese is toxic, but only in very high concentration. And it is also a necessary consituent is water.
Hence, according to the options available, best possible answer is (d)
Q1: Match the given water properties in Group-I to the given titrants shown in Group-ll. [2013 : 1 Mark]
(a) P-1, Q-2, R-3, S-4
(b) P-2, Q-1, R-4, S-3
(c) P-3, Q-4, R-1, S-2
(d) P-4, Q-3, R-2, S-1
Ans: (c)
Q1: A water sample has a pH of 9.25. The concentration of hydroxyl ions in the water sample is [2012 : 2 Marks]
(a) 10-9.25moles/L
(b) 10-4.75 moles/L
(c) 0.302 mg/L
(d) 3.020 mg/L
Ans: (c)
Sol: pH = 9.25
∵ pH + pOH = 14
⇒ pOH = 1 4 - 9 . 2 5 = 4.75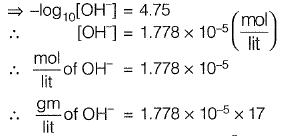
= 30.226 x 10-5
= 30.226 x 10-6 gm/lit
∴ [OH-] = 302.6 x 10-3 mg/lit
= 0.302 mg/lit
Q1: Anaerobically treated effluent has MPN of total coliform as 106/100 mL. After chlorination, the MPN value declines to 102/100 mL. The percent removal (%R) and log removal (log R) of total coliform MPN is [2011 : 1 Mark]
(a) %R = 99.90; log R = 4
(b) %R = 99.90; log R = 2
(c) % R = 99.99; log R = 4
(d) % R = 99.99; log R = 2
Ans: (c)
Sol:
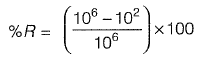
= 0.9999 x 100 = 99.99%
log R = log 106 - log 102
= 6 - 2 = 4
Q1: Ion concentrations obtained for a groundwater sample (having pH = 8.1) are given below
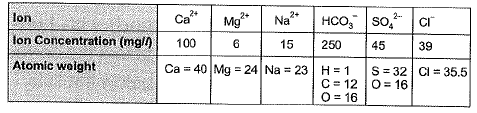
Carbonate hardness (mg/L as CaCO3) present in the above water sample is [2010 : 2 Marks]
(a) 205
(b) 250
(c) 275
(d) 289
Ans: (a)
Sol: Total alkalinity in water consists of alkalinity caused by
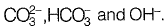 A little negative alkalinity is also caused by H+.
A little negative alkalinity is also caused by H+.∴ Total Alkalinity
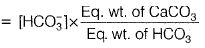
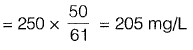
Carbonate hardness is equal to the total hardness or alkalinity, whichever is less. The carbonate hardness of the sample will be 205 mg/L Non-carbonate hardness
= Total hardness - carbonate hardness
= 275 - 205 = 70 mg/L
Q2: Ion concentrations obtained for a groundwater sample (having pH = 8.1) are given below

Total hardness (mg/L as CaCO3) present in the above water sample is [2010 : 2 Marks]
(a) 205
(b) 250
(c) 275
(d) 308
Ans: (c)
Sol: Total hardness
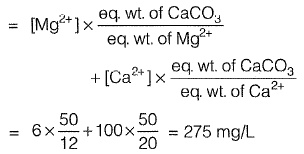
|
14 videos|99 docs|98 tests
|
FAQs on Past Year Questions: Quality Characteristics of Water - Environmental Engineering - Civil Engineering (CE)
| 1. What are the main quality characteristics of water? |  |
| 2. Why is pH important in determining water quality? |  |
| 3. How does turbidity affect water quality? |  |
| 4. What are common biological indicators of water quality? |  |
| 5. How can human activities impact water quality? |  |






















