R, S Nomenclature of Enantiomers, Diastereomers & Topicity | Organic Chemistry PDF Download
Why do we need R and S nomenclature?
Since enantiomers are two different compounds, they need to be distinguished by name. This is done by adding the prefix R or S to the IUPAC name of the enantiomer. Naming enantiomers with the prefixes R or S is called the Cahn-Ingold-Prelog system.
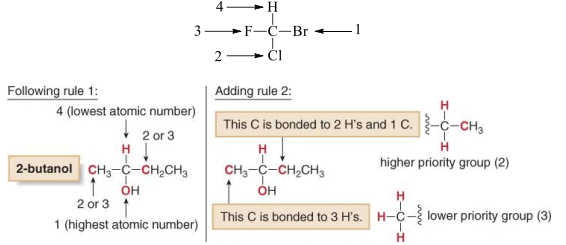
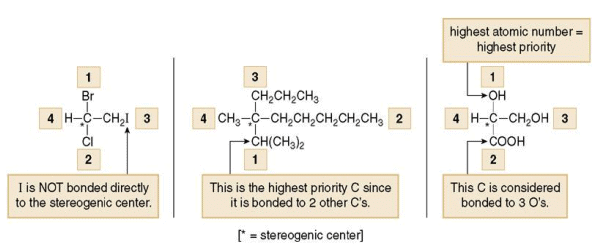
1. To designate enantiomers as R or S, priorities must be assigned to each group bonded to the stereogenic center, in order of decreasing atomic number. The atom of highest atomic number gets the highest priority (1).
2. If two isotopes are bonded to the stereogenic center, assign priorities in order of decreasing mass number. Thus, in comparing the three isotopes of hydrogen, the order of priorities is:

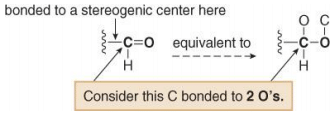
3. To assign a priority to an atom that is part of a multiple bond, treat a multiply bonded atom as an equivalent number of singly bonded atoms. For example, the C of a C=O is considered to be bonded to two O atoms.
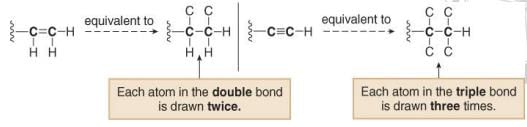
Other common multiple bonds are drawn below:
4. Hydrogen is the lowest priority group when lone pair is absent. When lone pair is present it is given the lowest priority.
5. ‘Cis’ precedes ‘trans’ and Z precedes E
6. Like pair R,R or S,S precedes unlike pair R,S or S,R
7. R precedes S; M precedes P
How to assign the R and S configuration?
1. Assign the priority.
2. Draw an arrow from the 1st priority group to the 2nd group to the 3rd group.
3. Clockwise rotation gives R configuration.
4. Anti-clockwise rotation gives S configuration.
[Note that 4th priority group should always be vertically upward/downward, if not, simply change the finally obtained configuration to other one].
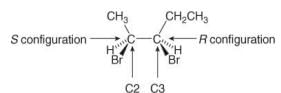
R and S Assignments in Compounds with Two or More Stereogenic Centers
When a compound has more than one stereogenic center, R and S configurations must be assigned to each of them.

Chemical Properties of Enantiomers
- Two enantiomers have exactly the same chemical properties except for their reaction with chiral non-racemic reagents.
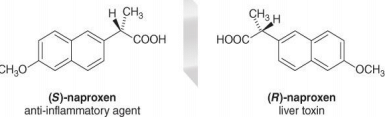
- Many drugs are chiral and often must react with a chiral receptor or chiral enzyme to be effective. One enantiomer of a drug may effectively treat a disease whereas its mirror image may be ineffective or toxic.
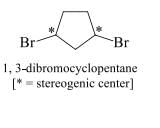
Diastereomers: Stereoisomers that are not mirror images of one another are called diastereomers.Consider a disubstituted cycloalkane.
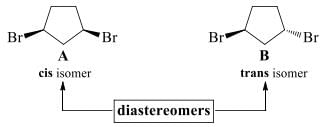
- It can have two substituents on the same side of the ring (cis isomer, A) or on opposite sides of the ring (trans isomer, B). These compounds are stereoisomers but not mirror images.
Epimers: When two Diastereomers differ in the stereochemistry at only one stereocenter then these are called Epimers. The term is quite general. However it is not used for molecules with only two stereocenters. Glucose and mannose are Epimers at C2.
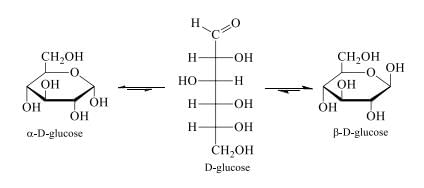
Anomers: α-D-Glucose and β-D-glucose are anomers. Anomers are two sugars that differ in configuration only at the carbon that was the carbonyl carbon in the open–chain form. This carbon is called the Anomeric carbon. The prefixes α- and β- denote the configuration about the anomeric carbon. Because anomers, like epimers, differ in configuration at only one carbon, they too are a particular kind of Diastereomer. Notice that the anomeric carbon is the only carbon in the molecule that is bonded to two
oxygens.
Topicity
Stereochemical relationships between individual atoms or groups or faces within a single molecule can be defined in terms of topicity.
Prochirality
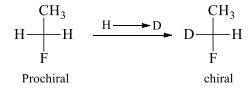
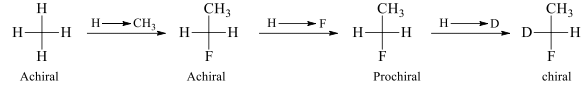
If changing one of two same groups by another group leads to chiralty, then, molecule is said to have prochirality or molecule is prochiral.
Homomer
The molecules which are same but appear different are called homomers.
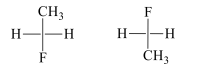
Homomers are generally Prochiral molecules. Homomers are equivalent structure of the same molecule.
Homotopic: Two atoms in equivalent environments are homotopic. e.g. the methylene protons in n-propane, two faces of carbonyl group in acetone
Homotopic ligands
Two ligands in an achiral molecule if on replacement yields homomer then they are termed as homotopic ligands.
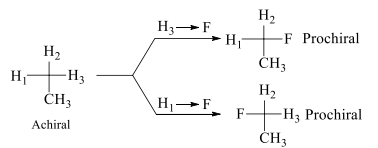
In the above shown figure H1, H2, H3 are homotopic ligands. These ligands are exchanged with the help of simple rotational axis of any fold.
Homotopic faces
Two faces in an achiral molecule are homotopic faces if attack from each side yields homomer.
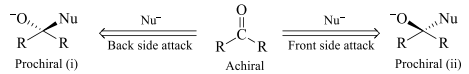
In the above shown figure i & ii are homomers so both the faces of the given compound are homotopic faces.
Enantiotopic:
Two ligands (or faces) equated by a mirror reflection of the molecule are enantiotopic e.g.
methylene protons of ethanol, two faces of carbonyl group in aldehyde (other than formaldehyde!)
Enantiotopic ligands
In a prochiral molecule, two ligands which are same are called enantiotopic ligands if an alternative interchange of them yields enantiotopic compounds / enantiomers
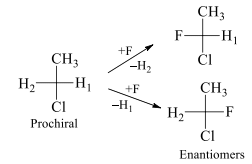
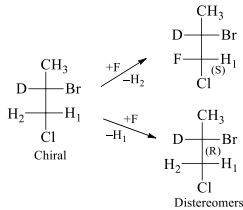
H1 and H2 are enantiotopic ligands. On exchanging these enantiotopic ligands with F it results into the conversion of prochiral molecule into chiral molecules which are non-superimposable mirror images of each other and hence called enantiomers. In this same molecule the hydrogen atoms of methyl group are homotopic because on replacement of each hydrogen with other group we get the same configuration molecule. These ligands are exchanged with plane of symmetry.
Enantiotopic faces
In an achiral molecule if two faces on attack by any group (nucleophile) yields enantiomers then the faces are called enantiotopic faces.
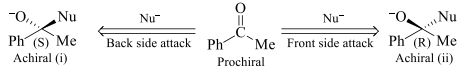
In the above shown figure i & ii are enantiomers so both the faces of the given compound are enantiotopic faces.
Diastereotopic: Two ligands placed in diasteromeric positions by a mirror reflection are diastereotopic. e.g. methylene protons in phenyl alanine, faces of carbonyl group in 4-tert-butylcyclohexanone.
Diastereotopic ligands
Two ligands are diastereotopic if alternative replacement of them yields diastereomers. These ligands can’t be exchanged by any type of symmetry element.
Diastereotopic faces
Two faces in a molecule are diastereotopic if attack from these sites yields diastereomers.
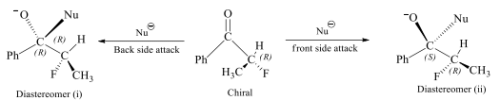
In the above shown figure i & ii are diastereomers so both the faces of the given compound are diastereotopic faces.
Nomenclature of faces and ligands
Faces are given Re or Si on the basis of the rules of CIP. Same rules are applied.

In the same way we can solve other problems related with nomenclature.
|
44 videos|102 docs|52 tests
|
FAQs on R, S Nomenclature of Enantiomers, Diastereomers & Topicity - Organic Chemistry
| 1. What is the nomenclature of enantiomers? |  |
| 2. How are diastereomers named? |  |
| 3. What is topicity in stereochemistry? |  |
| 4. How are enantiomers and diastereomers different? |  |
| 5. How is the configuration of enantiomers determined using the R, S system? |  |
















