Grade 11 Exam > Grade 11 Notes > Chemistry for Grade 11 (IGCSE) > Redox Reactions in term of Electron Exchange, Oxidation Number
Redox Reactions in term of Electron Exchange, Oxidation Number | Chemistry for Grade 11 (IGCSE) PDF Download
| Table of contents |

|
| Redox & Electron Transfer |

|
| Identifying Redox Reactions |

|
| Testing Redox Reactions |

|
| Oxidizing & Reducing Agents |

|
Redox & Electron Transfer
Oxidation & Reduction
- Redox reactions involve electron transfer between substances
- Oxidation refers to a reaction where an element, ion, or compound loses electrons, leading to an increase in its oxidation number. For instance, when silver reacts with chlorine, silver is oxidized to silver ions
Ag → Ag+ + e-
- Reduction is a chemical reaction where an element, ion, or compound gains electrons, leading to a decrease in its oxidation number. This process is often represented in a half equation.
- Example: When oxygen reacts with magnesium, oxygen is reduced to oxide ions.
O2 + 4e- → 2O2-
- Example: When oxygen reacts with magnesium, oxygen is reduced to oxide ions.
Example: Identifying Redox Reactions
- In the reaction between zinc and copper sulfate, zinc displaces copper from the compound, forming zinc sulfate and elemental copper.
- This reaction can be represented as: Zn + CuSO4 → ZnSO4 + Cu.
- The ions present in the equation include: Zn(s), Cu2+(aq), SO42-(aq), Zn2+(aq), SO42-(aq), Cu(s).
- The spectator ions, which do not participate in the reaction, are represented by SO42- (aq).
- These spectator ions can be removed, simplifying the equation to focus on the reactive species.
- Upon analyzing the ionic equation, it can be separated into two half equations by accounting for the transfer of electrons.
- Zn(s) → Zn2+(aq) + 2e-
- Cu2+(aq) + 2e- → Cu(s)
- Observing the half equations reveals that zinc undergoes oxidation (loses electrons), while copper ions experience reduction (gain electrons).
Identifying Redox Reactions
Oxidation Number
- The oxidation number (also known as oxidation state) represents the level of oxidation (or reduction) of an atom or ion within a compound.
- It signifies the number of electrons that an atom has either gained, lost, or shared during the formation of a compound.
- Oxidation numbers are crucial for monitoring electron movement in redox processes. They are denoted by a positive or negative sign followed by a number (unlike charges, which are represented by a number followed by a positive or negative sign).
- For instance, aluminum typically exhibits an oxidation state of +3 in compounds.
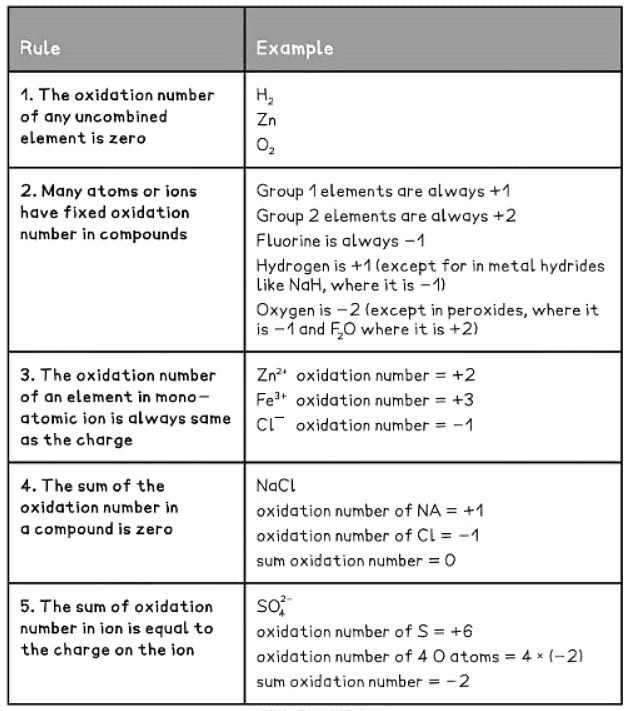
- There are straightforward rules to assist in determining the oxidation number of any element.
- Table of Rules for Assigning Oxidation Numbers:

- Redox reactions are recognized by changes in the oxidation numbers as reactants transform into products.
Testing Redox Reactions
- The examination of redox reactions involves observing a color alteration in the solution under analysis.
- Two prevalent examples include acidified potassium manganate(VII) and potassium iodide.
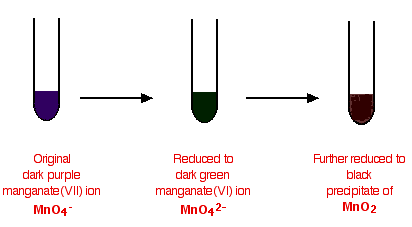
- Potassium manganate(VII) (KMnO4) functions as an oxidizing agent, commonly utilized to detect reducing agents.
- When acidified potassium manganate(VII) interacts with a reducing agent, its purple hue fades to colorless.
- Diagram illustrating the color shift when potassium manganate(VII) reacts with a reducing agent.

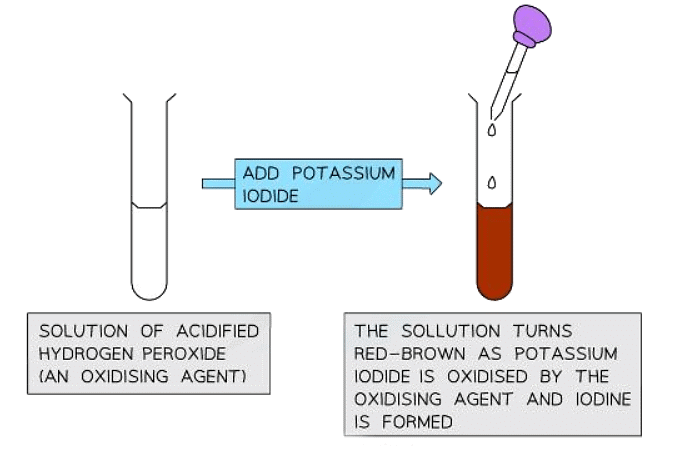
- Potassium iodide (KI) is a reducing agent commonly employed to identify oxidizing agents.
- When added to an acidic solution of an oxidizing agent like aqueous chlorine or hydrogen peroxide (H2O2), the solution adopts a red-brown color due to iodine (I2) formation.
2KI(aq) + H2SO4(aq) + H2O2(aq) → I2(aq) + K2SO4(aq) + 2H2O(l) - Potassium iodide acts as a reducing agent because it undergoes oxidation by losing electrons while hydrogen peroxide is reduced.
2I- → I2 + 2e- - Diagram to show the colour change when potassium iodide is added to an oxidising agent:

Oxidizing & Reducing Agents
Oxidizing Agent
- Definition: An oxidizing agent is a substance that causes another substance to be oxidized while itself getting reduced.
- Key terms: Oxidizes, Reduced
- Examples: Oxidizing agents include hydrogen peroxide, fluorine, and chlorine.
Reduction and Oxidation in Chemistry
- A reducing agent is a substance that reduces another substance and becomes oxidized in the process.
- Common examples of reducing agents include carbon and hydrogen.
- Reduction is crucial in the chemical industry for extracting metals from ores.
Example
- CuO + H2 → Cu + H2O
- In the given reaction, hydrogen is serving as the reducing agent, causing the reduction of CuO, while undergoing oxidation itself by losing electrons.
H2 → 2H+ + 2e- - Copper oxide (CuO) acts as the oxidizing agent, causing the oxidation of hydrogen, while itself being reduced to copper (Cu) by gaining electrons.
Cu2+ +2e- → Cu
Question for Redox Reactions in term of Electron Exchange, Oxidation NumberTry yourself: Which substance is acting as the reducing agent in the reaction between acidified potassium manganate(VII) and a reducing agent?View Solution
The document Redox Reactions in term of Electron Exchange, Oxidation Number | Chemistry for Grade 11 (IGCSE) is a part of the Grade 11 Course Chemistry for Grade 11 (IGCSE).
All you need of Grade 11 at this link: Grade 11
|
103 docs|53 tests
|
FAQs on Redox Reactions in term of Electron Exchange, Oxidation Number - Chemistry for Grade 11 (IGCSE)
| 1. What is the significance of identifying redox reactions? |  |
Ans. Identifying redox reactions is important because it helps us understand the transfer of electrons between reactants, which is crucial in various chemical processes and reactions.
| 2. How can one test for redox reactions in a chemical reaction? |  |
Ans. Redox reactions can be tested by examining changes in oxidation numbers of elements involved in the reaction, observing the transfer of electrons, or detecting the formation of new substances.
| 3. What are some common oxidizing and reducing agents in redox reactions? |  |
Ans. Common oxidizing agents include oxygen, hydrogen peroxide, and chlorine, while common reducing agents include metals like zinc and sodium, as well as carbon monoxide.
| 4. How do redox reactions relate to electron exchange and oxidation numbers? |  |
Ans. In redox reactions, electrons are transferred between reactants, leading to changes in oxidation numbers of the elements involved. The element being oxidized loses electrons and increases its oxidation number, while the element being reduced gains electrons and decreases its oxidation number.
| 5. How can the concept of redox reactions be applied to real-life situations? |  |
Ans. Redox reactions play a crucial role in various processes, such as corrosion of metals, photosynthesis in plants, and respiration in living organisms. Understanding redox reactions can help in predicting and controlling chemical reactions in these contexts.
Related Searches