Reversible & Irreversible Processes | Physics for EmSAT Achieve PDF Download
Reversible Process
A thermodynamic process is reversible if the process can return back in such a that both the system and the surroundings return to their original states, with no other change anywhere else in the universe. It means both system and surroundings are returned to their initial states at the end of the reverse process.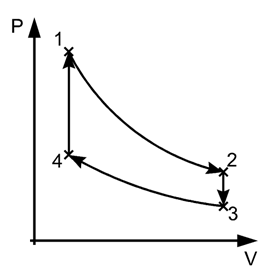
In the figure above, the system has undergone a change from state 1 to state 2. The reversible process can reverse completely and there is no trace left to show that the system had undergone thermodynamic change. During the reversible process, all the changes in state that occur in the system are in thermodynamic equilibrium with each other.
Internally reversible process
The process is internally reversible if no irreversibilities occur within the boundaries of the system. In these processes, a system undergoes through a series of equilibrium states, and when the process reverses, the system passes through exactly the same equilibrium states while returning to its initial state.
Externally reversible process
In externally reversible process no irreversibilities occur outside the system boundaries during the process. Heat transfer between a reservoir and a system is an externally reversible process if the surface of contact between the system and reservoir is at the same temperature.
A process can be reversible only when its satisfying two conditions
- Dissipative force must be absent.
- The process should occur in infinite small time.
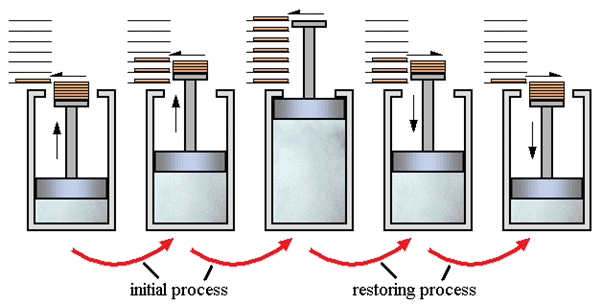
In simple words, the process which can reverse back completely is a reversible process. This means that the final properties of the system can perfectly reverse back to the original properties. The process can be perfectly reversible only if the changes in the process are infinitesimally small. In practical situations it is not possible to trace these extremely small changes in extremely small time, hence the reversible process is also an ideal process. The changes that occur during the reversible process are in equilibrium with each other.
Irreversible Process
Irreversible processes are a result of straying away from the curve, therefore decreasing the amount of overall work done. An irreversible process is a thermodynamic process that departs from equilibrium. In terms of pressure and volume, it occurs when the pressure (or the volume) of a system changes dramatically and instantaneously that the volume (or the pressure) do not have the time to reach equilibrium.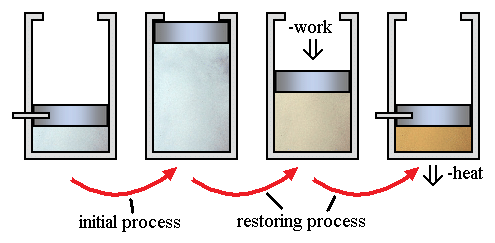
A classic example of an irreversible process is allowing a certain volume of gas to release into a vacuum. By releasing pressure on a sample and allowing it to occupy a large space, the system and surroundings are not in equilibrium during the expansion process.
Here little work occurs. However, there is a requirement of significant work, with a corresponding amount of energy dissipation as heat flows to the environment. This is in order to reverse the process.
|
208 videos|329 docs|212 tests
|
















