SSS 2 Exam > SSS 2 Notes > Chemistry for SSS 2 > Revision Notes: Air
Revision Notes: Air | Chemistry for SSS 2 PDF Download
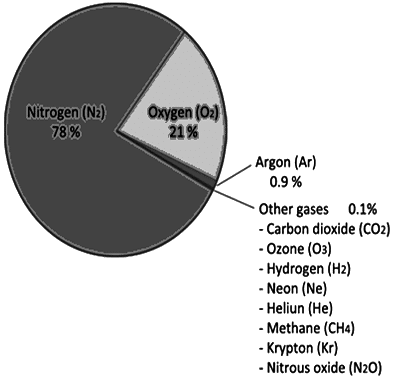
- Nitrogen: dilutes effect of oxygen and controls rate of combustion Needed for proteins and important compounds like fertilizers, ammonia and nitric acid Used to keep food fresh because it does not allow growth of oxygen by preventing oxidation
- Oxygen: needed to sustain life, and for combustion
- Carbon dioxide: needed for photosynthesis, retains heat in atmosphere and balances temperature on earth. Used in fire extinguishers
- Inert gases: Helium is used in meteorological balloons, Neon is used in light bulbs, Radon is used in treatment of cancer. Krypton and Xenon are used in photography
Dust particles and water vapours help in formation of clouds
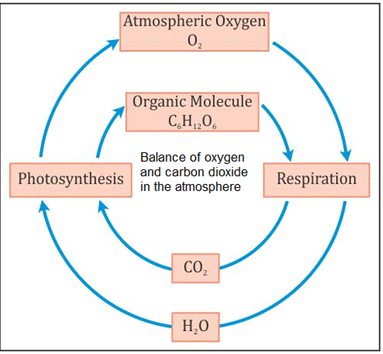
Balance of Oxygen and Carbon Dioxide in Nature
Rusting needs air and moisture. Can be prevented by painting, galvanization and alloying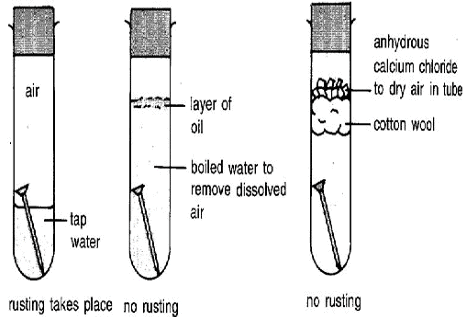
Burning or Combustion
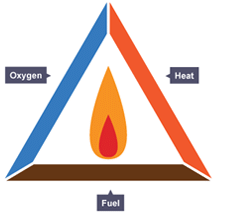
- A fire needs air/oxygen
- A combustible substance (fuel)
- Right temperature
- Burning is an exothermic reaction in which a fuel burns in oxygen to give light and heat energy
The document Revision Notes: Air | Chemistry for SSS 2 is a part of the SSS 2 Course Chemistry for SSS 2.
All you need of SSS 2 at this link: SSS 2
|
1 videos|45 docs|16 tests
|
FAQs on Revision Notes: Air - Chemistry for SSS 2
| 1. What is air and why is it important for life on Earth? |  |
Ans. Air is a mixture of gases that surround the Earth, primarily composed of nitrogen (78%) and oxygen (21%), along with trace amounts of other gases like carbon dioxide and argon. It is essential for life as it provides the oxygen needed for respiration in animals and humans, and it supports photosynthesis in plants, which produces the oxygen we breathe.
| 2. How does air pressure affect weather and climate? |  |
Ans. Air pressure is the weight of the air above us and it plays a crucial role in weather patterns. High pressure usually leads to clear, sunny skies, while low pressure is often associated with clouds and precipitation. Changes in air pressure can cause wind, storms, and other weather phenomena, influencing the climate of a region.
| 3. What are the components of air and how do they affect our environment? |  |
Ans. The main components of air are nitrogen, oxygen, carbon dioxide, argon, and water vapor. Nitrogen is inert and does not react easily, while oxygen is vital for combustion and respiration. Carbon dioxide is important for plant growth but can contribute to global warming in excess. Water vapor is crucial for weather and climate, affecting humidity and precipitation.
| 4. How do plants and animals depend on air? |  |
Ans. Plants rely on air for the carbon dioxide they need for photosynthesis, which is the process they use to create food. Animals, including humans, depend on air for oxygen, which is essential for cellular respiration. This interdependence highlights the critical role air plays in maintaining life on Earth.
| 5. What methods can be used to measure air quality? |  |
Ans. Air quality can be measured using various methods, including air sampling and analysis of pollutants like particulate matter, sulfur dioxide, carbon monoxide, and ozone. Instruments such as air quality monitors and sensors can provide real-time data on air pollution levels, helping to assess the health of the environment and inform public health decisions.
Related Searches















