SSS 2 Exam > SSS 2 Notes > Chemistry for SSS 2 > Revision Notes: Periodic Table - Periodic Properties and Variations of Properties
Revision Notes: Periodic Table - Periodic Properties and Variations of Properties | Chemistry for SSS 2 PDF Download
Introduction
- It is a table which classifies all the known elements in accordance with their properties in such a way that elements with similar properties are grouped together in the same vertical column and dissimilar elements are separated.
- The 115 known elements are arranged in the Periodic Table in order of their increasing atomic number.
- The vertical columns are called groups.
- The horizontal rows are called periods.
Approaches to Periodic Classification of Elements
Dobereiner’s Triads
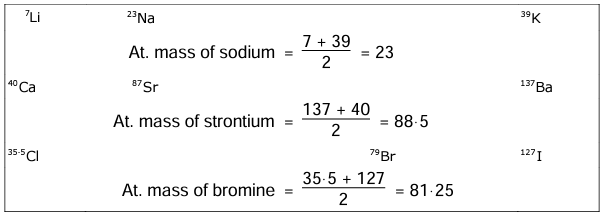
In 1817, Dobereiner classified elements with similar chemical properties into groups of three called triads. He noted that the atomic mass of the middle element in a triad is the arithmetic mean of the other two.
This is called Dobereiner’s Law of Triads.
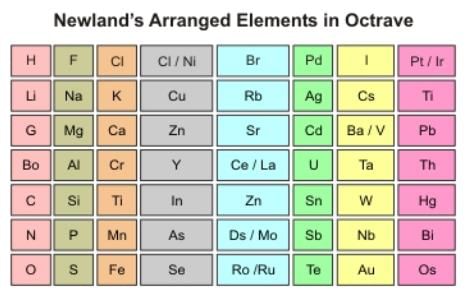
Newlands’ Law of Octaves
In 1864, Newlands arranged elements in the increasing order of atomic mass and noticed that the eighth element was similar in physical and chemical properties to the first element, just like the eight nodes in music. This relationship is called the Law of Octaves.
Lother Meyer’s Law
In 1869, Lother Meyer classified that atomic volume is the periodic function of atomic mass.
Mendeleev’s Periodic Law
In 1869, Mendeleev stated the law of chemical periodicity.
The physical and chemical properties of elements are a periodic function of their atomic masses; i.e. when the elements are arranged in the increasing order of their atomic masses, the elements with similar properties are repeated after certain regular intervals.
This is called Mendeleev’s Periodic Law.
Certain anomalies of Mendeleev’s table were
(i) Position of hydrogen
(ii) Position of rare earths
(iii) Position of isotopes
(iv) Position of Noble gases
(v) Anomalous pairs of elements
(vi) Cause of periodicity
Modern Periodic Law
The physical and chemical properties of elements are a periodic function of their atomic numbers.Long Form of the Periodic Table
It is based upon the modern periodic law, i.e. elements are arranged according to the increasing order of their atomic number.
Periods
- The horizontal rows of elements in a periodic table are called periods. There are seven periods in the long form of the periodic table.
- The first period consists of 2 elements called the shortest period.
- The second and third periods consist of 8 elements called short periods.
- The fourth and fifth periods contain 18 elements each and are called long periods.
- The sixth period consists of 32 elements and it is the longest period.
- The seventh period is yet an incomplete period.
Groups
- The modern periodic table has eighteen vertical columns known as groups, arranged from left to right in the order: IA, IIA, IIIB, IVB, VB, VIB, VIIB, VIII (three columns), IB, IIB, IIIA, IVA, VA, VIA, VIIA and Zero.
- A group is determined by the number of electrons present in the outermost shell.
- Elements in groups 1, 2 and 13 to 17 are called normal elements.
- Elements in groups 3 to 12 are called transition elements.
- Group 18 at the extreme right contains noble or inert gases.
- Reactive metals are placed in groups 1 and 2.
- Transition elements [metals] are placed in the middle.
- Non-metals are placed in the upper right corner of the periodic table.
Periodicity
The properties which reappear at regular intervals, or in which there is gradual variation at regular intervals, are called periodic properties, and the phenomenon is known as the periodicity of elements.
Shells/Orbits
Electrons in an atom revolve around the nucleus in certain selected but fixed concentric circular paths called shells or orbits. These are associated with a definite amount of energy and are also called energy levels.
Valency
- It denotes the combining capacity of the atom of an element. It is equal to the number of electrons an atom can donate or accept or share.
- On moving from left to right in a period, the number of valence electrons increases from 1 to 8.
- Certain elements lose electrons in steps and hence show variable valency, e.g. Cu, Fe, Ag, Au etc.
- On moving down a group, the valence electrons and valency of all the elements in a group remain the same.
Periodic Properties of Elements
- Atomic size (atomic radii): It is the distance between the centre of the nucleus of an atom and its outermost shell.
- Metallic Character: Those elements which have a tendency to lose their valence electrons and form a positive ion are considered as metals.
Na − e− → Na+ - Non-metallic Character: Those elements which have a tendency to gain electrons in order to attain an octet in their outermost orbit are considered as non-metals.
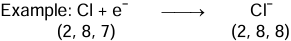
- Chemical Reactivity: In metals, greater the tendency to lose electrons, greater is the reactivity.
In non-metals, greater the tendency to gain electrons, greater is the reactivity. - Ionisation Energy: The energy required to remove an electron from a neutral isolated gaseous atom and convert it into a positively charged gaseous ion is called ionisation energy (IE) or first ionisation energy (IE1).
- Electron Affinity (EA) or Electron Gain Enthalpy: The amount of energy released while converting a neutral gaseous isolated atom into a negatively charged gaseous ion by the addition of electrons is called electron affinity.
- Electronegativity: The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is called its electronegativity.
- Atomic Number (Z): The atomic number of an element is equal to the number of protons in the nucleus.
The atomic number is a unique property of an element, because no two elements have the same atomic number. - Mass Number (A): The mass number of an element is the sum of the number of protons and neutrons in the nucleus of the atom of the element.
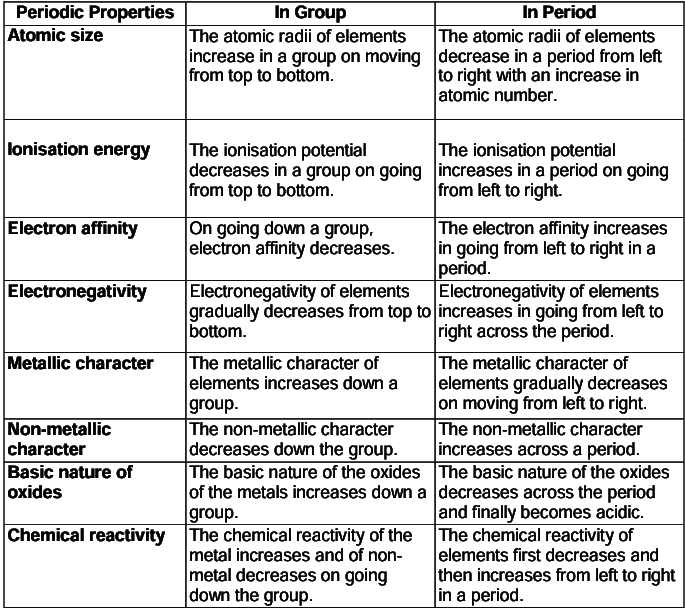
Variation of Periodic Properties in the Periodic Table
The document Revision Notes: Periodic Table - Periodic Properties and Variations of Properties | Chemistry for SSS 2 is a part of the SSS 2 Course Chemistry for SSS 2.
All you need of SSS 2 at this link: SSS 2
|
1 videos|45 docs|16 tests
|
FAQs on Revision Notes: Periodic Table - Periodic Properties and Variations of Properties - Chemistry for SSS 2
| 1. What are Dobereiner’s Triads and how do they relate to the periodic classification of elements? |  |
Ans. Dobereiner’s Triads are groups of three elements that have similar chemical properties, where the atomic mass of the middle element is approximately the average of the atomic masses of the other two elements. This concept was an early attempt to classify elements based on their properties and laid the groundwork for later periodic classifications. For example, in the triad of lithium, sodium, and potassium, sodium has an atomic mass that is roughly the average of lithium and potassium. This idea highlighted the relationship between atomic mass and chemical properties.
| 2. What is Newlands’ Law of Octaves and its significance in the periodic table? |  |
Ans. Newlands’ Law of Octaves states that when elements are arranged in order of increasing atomic mass, every eighth element exhibits similar properties, akin to musical octaves. This was significant because it was one of the first attempts to establish a systematic arrangement of elements. However, it had limitations, as it only applied to lighter elements and failed for heavier elements, but it paved the way for more refined periodic laws.
| 3. How does Mendeleev’s Periodic Law differ from the Modern Periodic Law? |  |
Ans. Mendeleev’s Periodic Law states that the properties of elements are a periodic function of their atomic masses, meaning that elements with similar properties recur at regular intervals when arranged by increasing atomic mass. In contrast, the Modern Periodic Law states that the properties of elements are a periodic function of their atomic numbers, which is the number of protons in the nucleus. The Modern Periodic Law corrects the inconsistencies found in Mendeleev’s arrangement and accounts for the discovery of isotopes.
| 4. What are the key features of the Long Form of the Periodic Table? |  |
Ans. The Long Form of the Periodic Table organizes elements into periods and groups based on their electronic configuration. It includes s, p, d, and f blocks, reflecting the filling of electron shells. This form clearly shows the periodic trends and properties of elements, such as atomic size, ionization energy, and electronegativity, making it easier to understand the relationships between different elements. It also accommodates the lanthanides and actinides as separate rows.
| 5. What is meant by periodicity in the context of the periodic table? |  |
Ans. Periodicity refers to the recurring trends that are observed in the properties of elements when they are arranged in the periodic table. These trends include variations in atomic size, ionization energy, electronegativity, and metallic character across periods and down groups. Periodicity arises due to the regular arrangement of elements based on their atomic structure and electron configuration, allowing for predictions about the behavior of elements based on their position in the table.
Related Searches
















