Separation of Substances Class 6 Worksheet Science Chapter 3
Q1: True or False
i. The property used in separating a mixture of two solids by winnowing is the difference in weight.
Ans: True
Winnowing separates lighter components like husk from heavier grains using wind or air.
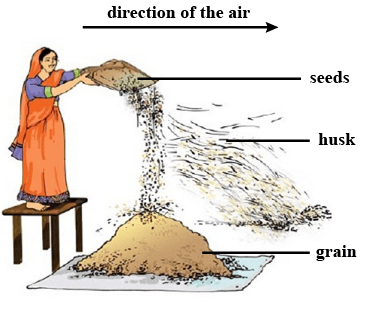 Winnowing
Winnowing
ii. A mixture of milk and water can be separated by filtration.
Ans: False
Filtration is ineffective for separating milk from water since both are liquids and the particles are too small.
iii. A mixture of powdered salt and sugar can be separated by the process of winnowing.
Ans: False
Winnowing is used for separating components with significant differences in weight, which is not applicable to salt and sugar.
iv. Separation of sugar from tea can be done with filtration.
Ans: False
Sugar dissolves in tea, so filtration cannot separate it.
v. Grain and husk can be separated with the process of decantation.
Ans: False
Decantation separates solids from liquids, while grain and husk are separated by winnowing.
vi. Sieving is used when the components of the mixture are of different sizes.
Ans: True
Sieving separates components based on size, like separating bran from flour.
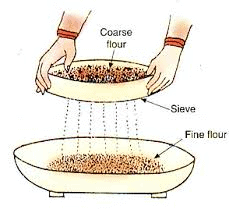 Seiving
Seiving
vii. The method of filtration is also used in the process of preparing cottage cheese in homes.
Ans: True
Filtration separates solid paneer from liquid whey during the preparation of cottage cheese.
viii. When no more salt can be dissolved in the given amount of water at a particular temperature, the solution is said to be unsaturated.
Ans: False
The solution is said to be saturated, not unsaturated.
ix. Centrifugation is used to separate cream from curd.
Ans: True
Centrifugation separates components based on density, which is used to separate cream from curd.
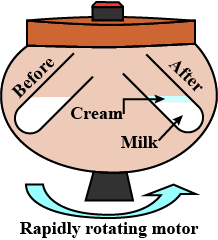 Centrifugation
Centrifugation
x. Hand-picking can be used to separate cashew nuts from a mixture of almonds and cashew nuts.
Ans: True
Hand-picking is effective for separating larger, easily distinguishable items like nuts.
Q2: Match the Following
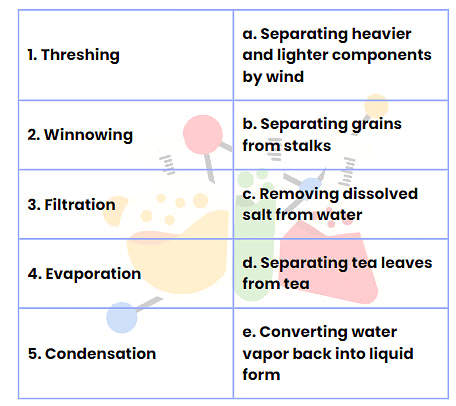
Ans: Here is the correct matching for the provided options:
- Threshing - b. Separating grains from stalks
- Winnowing - a. Separating heavier and lighter components by wind
- Filtration - d. Separating tea leaves from tea
- Evaporation - c. Removing dissolved salt from water
- Condensation - e. Converting water vapor back into liquid form
This matches the different separation techniques with their correct descriptions.
Q3: Fill in the Blanks
i. The process of separating a liquid from solid sediment is called .
Ans: decantation
ii. The method of separating seeds of paddy from its stalks is called .
Ans: threshing
iii. When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example of .
Ans: filtration
iv. Salt is obtained from seawater by the process of .
Ans: evaporation
v. is used to remove impurities and bran from the flour.
Ans: Sieving
vi. The two liquids that do not mix with each other can be separated by .
Ans: decantation
vii. We see water drops under the plate that has been used to cover a container containing milk that has just been boiled. This is due to process of .
Ans: condensation

Assertion and Reason Questions
Q4: Assertion (A): The process of conversion of liquid water to its vapours by heating the liquid is called evaporation.
Reason (R): The process of conversion of water vapours to liquid by cooling the vapours is called condensation.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Ans: (b)
Both statements are correct:
- Assertion (A) correctly describes evaporation, where liquid water turns into vapor when heated.
- Reason (R) accurately describes condensation, where water vapor turns back into liquid when cooled.
However, R does not explain A directly. They describe different processes, which is why the answer is (b).
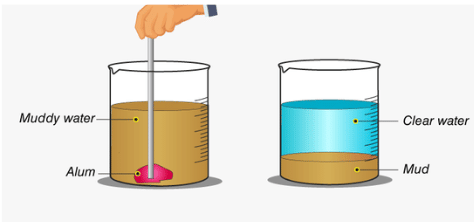
Q5: Assertion (A): The process of settling of heavier insoluble particles from a suspension of a substance in water known as decantation.
Reason (R): Decantation process along with sedimentation is used to get clear water from muddy water.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Ans. (d)
The assertion states that the process of settling heavier particles in water is called decantation, but this is incorrect. The correct term for settling heavier particles is sedimentation. Decantation is the process of carefully pouring off the clear liquid after sedimentation.
The reason (R) is true because sedimentation and decantation are used together to get clear water from muddy water.
Since the assertion is false and the reason is true, the correct answer is (c).
Answer the following
Q6: Write any two methods used for separation of substances.
Ans: Hand-picking and Threshing
Q7: How can we separate sand from water?
Ans: We can separate sand from water by using sedimentation to let the sand settle at the bottom, followed by decantation to pour off the clear water.
Q8: What happens when you continue to add salt to a fixed amount of water, and how can you increase the amount of salt that dissolves in water after it becomes saturated?
Ans: When you continue to add salt to a fixed amount of water, the water eventually becomes saturated, meaning no more salt can dissolve, and the excess salt will settle at the bottom. To increase the amount of salt that dissolves in the water after it becomes saturated, you can heat the water. Heating increases the water's capacity to dissolve more salt.
Q9: Which method would you prefer to separate solid dissolved in liquid?
Ans: Evaporation
Q10: Filtration method is used to separate tea leaves from prepared tea. Which other method can be used?
Ans: Decantation
Q11: List various methods of separation of components from their mixtures.
Ans: Some of the methods are: handpicking, winnowing, threshing, sedimentation, decantation, filtration, evaporation and condensation.
Q12: The process of adding alum to water to fasten sedimentation is called loading. Why has this name been given to the process?
Ans: The term "loading" is used because adding alum makes the suspended particles heavier, causing them to settle faster.
Q13: How can we separate oil and water from their mixture?
Ans: Use a separating funnel to drain the water from the oil-water mixture. The oil floats on top and can be left behind after draining the water.
Q14: Why are we able to dissolve more solute in a solvent at high temperature?
Ans: We are able to dissolve more solute in a solvent at a high temperature because high temperature facilitates dissolving reaction by providing energy to break bonds in the solid.
Q15: Why sieving is not used to separate very small stones from rice grains?
Ans. Sieving is not used to separate very small stones from rice grains because both are of almost the same size and both will pass through the holes of the sieve.
Q16: There are 3 beakers half filled with water. Now add 3 spoons of sugar in the first beaker, 5 spoons of sugar in the second beaker and 7 spoons of sugar in the third beaker. Stir the solution of all three beakers. Which solution is more saturated?
Ans: The solution in the third beaker is more saturated because it contains the most sugar (7 spoons) compared to the other beakers.
Q17: What is winnowing? Where is it used?
Ans: Winnowing is the method in which heavier components of the mixture are separated from the lighter components such as chaff, dirt, etc. by dropping the mixture from height into the air. This method is used by farmers to separate husk and other lighter impurities from grains.
Q18: Why do we separate substances?
Ans: We need to separate the components of a mixture for the following reasons:
- To separate two different but useful components from the mixture.
- To remove non–useful components from the mixture.
- To remove impurities or harmful substances from the mixture.
- To remove the unwanted impurities from the mixture.
- To obtain pure substances by removing the other substances.
Q19: How will you separate husk or dirt particles from a given sample of pulses before cooking?
Ans: Husk or dirt particles from a given sample of pulses can be removed by washing the pulses with water. Being heavier, pulses will settle down in the bottom of the container whereas lighter particles will keep floating in the water. This is called sedimentation. Dirty water can be removed by the process of decantation by leaving behind pulses in the bottom of the container.
Q20: What is sieving? Where is it used?
Ans: Sieving is a simple and convenient technique of separating particles of different sizes by allowing the smaller particles to pass through the holes of a sieve leaving the bigger particle in the sieve.
Uses of sieving
- Used in homes to separate flour from impurities such as husk, stalks and small pieces of stones.
- Used in flour mill to separate broken particles of grain from flour.
- Used at the construction sites to separate small pieces of stones from sand.
FAQs on Separation of Substances Class 6 Worksheet Science Chapter 3
| 1. What are the different methods used for the separation of substances? |  |
| 2. How does filtration work in separating substances? |  |
| 3. Can you explain the process of distillation for separating substances? |  |
| 4. What is chromatography and how is it used for separation? |  |
| 5. How does magnetism help in the separation of substances? |  |






















