Short & Long Answer Questions: Hydrocarbons | Organic Chemistry for NEET PDF Download
Q.1 In cracking, C-C bonds cleave in preference to C-H bonds. Explain.
Answer: The bond dissociation enthalpy of C-C bond (355kJmol−1) is less as compared to that of C-H bond(414kJmol−1). Therefore, in the cracking of alkanes, generally C-C bonds cleave to form smaller molecules.
Q.2 Branched chain alkanes have less boiling points as compared to straight chain isomers. Discuss.
Answer: In alkanes, the main attractive forces present in the molecules are weak van der Waals' forces which are linked with the surface area or the size of the molecule. The branching of the carbon atom chain brings the atoms closer, thereby decreasing the surface area and molecular size. Therefore, the branching of the carbon atom chain decreases the magnitude of the attractive forces. As a result, the branched chain alkanes have less boiling points as compared to straight chain isomers. For example,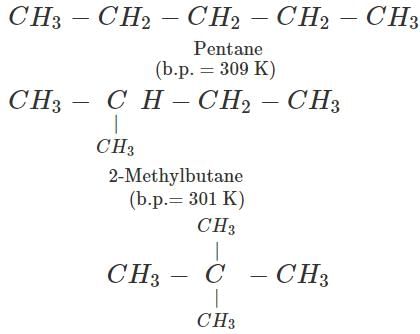
Q.3 An oxidising agent is needed in the iodination of methane but not in the chlorination or bromination. Assign reason.
Answer: In the iodination of methane, H−I is also formed as the product along with iodomethane. Since it is a strong reducing agent, it reduces iodomethane back to methane and makes the reaction reversible. In order to destory HI, oxidising agent like HIO3 (or HNO3) is needed. But HCl and HBr formed in the chlorination and bromination reactions of methane are not in a position to react with the mono substituted product (i.e., CH3−Cl and CH3−Br) since they are comparatively weak reducing agents. Therefore, no oxidising agent is needed for these reactions.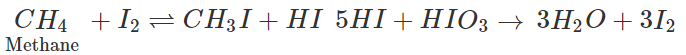
Q.4 Reaction with bromine dissolved in CCl4 or bromine water is used as a test for unsaturation both for alkenes and alkynes. Why?
Answer: Bromine is yellowish brown in colour. When vapours of an unsaturated hydrocarbon (alkene or alkyne) are passed through the solution of bromine in carbon tetrachloride or water, the product formed is colourless. Thus, the colour of bromine gets discharged. Therefore, the reaction is-
Q.5 When methane is chlorinated, the products formed also contains traces of chloroethane. How will you account for it?
Answer: We know that the chlorination of methane proceeds by free radical mechanism in which methyl free radical (C⋅H3) is formed. Two such free radicals may combine to give ethane which upon further chlorination gives chloroethane.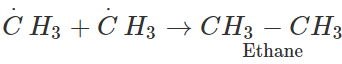
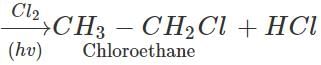
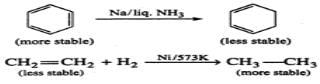
Q.6 Iodine does not react with ethane though I2 is more easily cleaved homolytically than the other halogens. Explain.
Answer: Bond dissociation enthalpy of I2 is less as compared to that of Cl2 and Br2 which means that the homolysis of I2 is the easiest. However, HI formed in a reaction is a strong reducing agent and it makes the reaction reversible. Therefore, iodine does not reaction with ethane.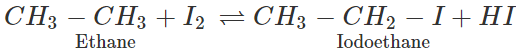
Q.7 An alkane with molecular formula C7H16 forms four monochloro products when heated with chlorine in the presence of ultra violet radiations. Give the structure of the alkane and also of the products. Write their IUPAC names as well.
Answer: The alkane which gives four isomeric monochloro products is 2,2-dimethylpentane. Since there are four types of carbon atoms present, there are four isomeric products. The products are given ahead.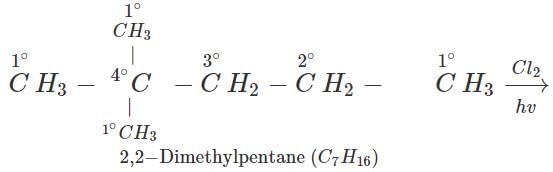
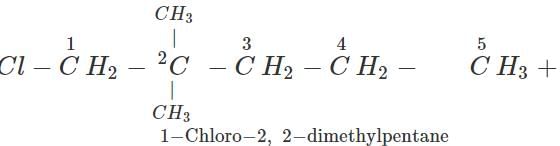
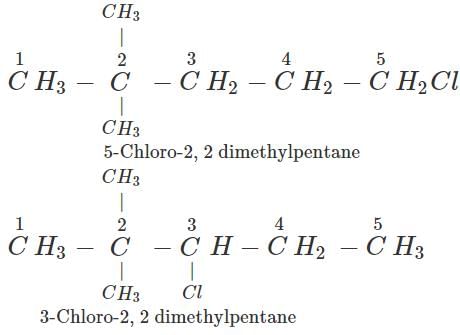
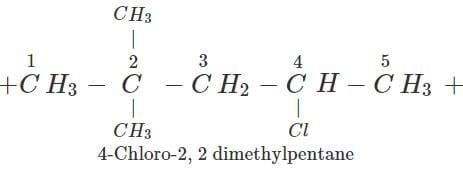
Q.8 Alkynes do not undergo ozonolysis like alkenes. Why?
Answer: Alkynes have a triple bond (C≡C) in their molecules. Reaction with ozone results in the formation of an ozonide. Now, treatment with zinc dust and water does not cleave the triple bond completely. Therefore, a mixture of two carbonyl compounds is not formed. In other words, alkynes do not undergo complete cleavage in the same way as alkenes.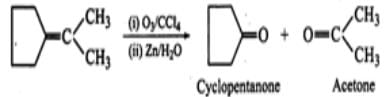
Q.9 Write all the isomeric cyclic structures for the molecular formula C7H8.
Answer: Four isomeric structures for the molecular formula are possible out of which three are isomeric xylenes while the fourth is ethylbenzene.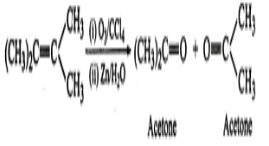

Q.10 The dipole moment of trans 1, 2-dichloroethene is less than the cis isomer. Discuss.
Answer: The structure of the trans isomer is more symmetrical as compared to the cis isomer. In the trans isomer, the dipole moments of the polar C−Cl bonds are likely to cancel with each other and the resultant dipole moment of the molecule is nearly zero. But in the cis isomer, these do not cancel. Therefore, the cis isomer has a specific dipole moment but is zero in case of trans isomer.
Q.11 An alkene has molecular mass 70. Write all the possible structural isomers and give their IUPAC names.
Answer: The general formula of alkene =CnH2n ∴ 12×n+1×2n=70, or 14n=70 or n=70/14=5. The molecular formula of alkene is C5H10. The possible structural isomers are: 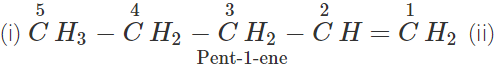
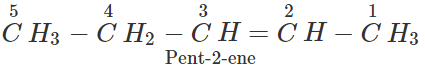
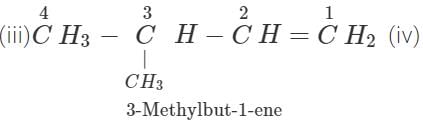
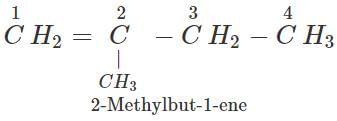
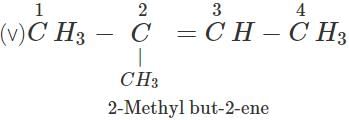
Q.12 Identify and justify the correct order of reactivity in electrophilic substitution in the following compounds:
Answer: The correct order of reactivity in electrophilic substitution is: 
The electrophile attack will be the easiest in case the ring is maximum activated out of the groups attached in different molecules. • ′CH3′ is highly activating due to hyper conjugation. • ′Cl′ is activating due to resonance (R) effect but at the same time it is deactivating due to−I effect. • ′NO2′is deactivating in nature. In the light of the above observations, the order of reactivity is justified.
Q.13 Out of but-1-yne and but-1-ene, which has more dipole moment?
Answer: The direction of the dipole moments as well as the hybridisation states of the carbon atoms are shown as follows: 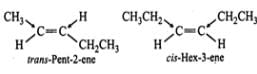
 The order of the electro negativity of the carbon atoms based on hybridisation issp>sp2>sp3 This means that CH3CH2−C≡C−(sp3−sp)bond is more polar than that of CH3CH2−CH=CH−(sp3−sp2) bond. Therefore, the dipole moment of but-1-yne is expected to be more than that of but-1-ene.
The order of the electro negativity of the carbon atoms based on hybridisation issp>sp2>sp3 This means that CH3CH2−C≡C−(sp3−sp)bond is more polar than that of CH3CH2−CH=CH−(sp3−sp2) bond. Therefore, the dipole moment of but-1-yne is expected to be more than that of but-1-ene.
Q.14 Give reasons for the following: (i) tert-butyl benzene does not give benzoic acid on treatment with acidic KMnO4 (ii)CH2= is more basic than HC ≡
is more basic than HC ≡ 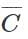
Answer: (i) Tertiary butyl benzene C6H5C(CH3)3 does not give benzoic acid on reacting with acidified KMnO4 because it does not contain a hydrogen atom attached directly to the carbon atom. (ii) We know that HC≡CH is a stronger acid (sp-hybridised carbon atoms) than H2C=CH2 (sp2-hybridised carbon atoms). The conjugate base 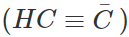 is, therefore, weaker than the conjugate base
is, therefore, weaker than the conjugate base 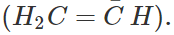
Q.15 Why does not aniline take part in the Friedel Craft reaction?
Answer: In aniline, the electron pair on the amino group 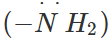 co-ordinates with AlCl3 (Lewis acid) to form a salt
co-ordinates with AlCl3 (Lewis acid) to form a salt 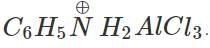 As a result, the ring is not properly activated and the Friedel Craft reaction does not take place.
As a result, the ring is not properly activated and the Friedel Craft reaction does not take place.
Q.16 Unsaturated compounds undergo addition reactions. Explain.
Answer: Unsaturation in the organic compounds is due to the presence of one or more double or triple bonds which contain π-bonds. Since these bonds are formed as a result of sidewise overlap, they are, therefore, weak. They can easily change to stable σ-bonds and this happens in the addition reactions. Therefore, unsaturated compounds take part in the addition reactions.
Q.17 But-1-yne is weakly acidic but but-2-yne is not. Assign reason.
Answer: But-1-yne (CH3−CH2−C≡C−H) is a terminal alkyne and the triple bonded carbon has a hydrogen atom attached to it which can be removed as a H+ by suitable reaction. On the other hand, but-2-yne (CH3−C≡C−CH3) is a non-terminal alkyne and the triple bonded carbon has no hydrogen atom attached to it. Therefore, it cannot behave as an acid.
Q.18 Which of the following has the highest boiling point? (i) 2-methylpentane (ii) 2, 3-dimethylbutane (iii) 2, 2-dimethylbutane.
Answer: As the branching increases, surface area decreases. As a result, magnitude of van der Waals forces of attraction decreases and hence the boiling point decreases. Now 2- methyl pentane has the largest surface area and hence has the highest boiling point. Further because of the presence of two branches on the same carbon, 2, 2-dimethylbutane has lower surface area and hence has lower boiling point than that of 2, 3-dimethylbutane. Thus, the overall order of decreasing boiling points is : 2-methylpentane (333 K) > 2, 3-dimethylbutane (331 K) > 2, 2- dimethylbutane (323 K).
Q.19 What effect the branching of an alkane has on its melting point?
Answer: In general, as the branching increases, the packing of the molecules in the crystal lattice becomes less close and hence the m.p. decreases accordingly. However, if the branching makes the molecule symmetrical, the packing of the molecules in the crystal lattice becomes close and hence the m.p. increases. Now since isopentane is less symmetrical than n-pentane but neopentane is the most symmetrical of the three isomeric pentanes, therefore, the m.p. of isopentane (113 K) is lower than of n- pentane (143 K) but the m.p. of neopentane (256 K) is much higher than that of isopentane and n-pentane.
Q.20 An organic compound C8H18 on mono chlorination gives a single mono chloride. Write the structure of the hydrocarbon.
Answer: Since, the hydrocarbon (C8H18) on mono chlorination gives a single mono chloride, therefore, all the 18 H-atoms are equivalent. The only such hydrocarbon is 2, 2, 3, 3-tetramethylbutane, i.e.,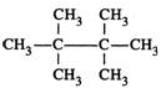
Q.21 What are the main constituents of LPG?
Answer: The main constituents of LPG are butane and isobutane. Both these isomers can be easily liquefied and hence can be conveniently transported in iron cylinders.
Q.22 Why do the C?C bonds rather than C?H bonds break during cracking of alkanes?
Answer: Since the bond dissociation energy of C?C bonds (348kJmol−1), is lower than bond dissociation energy of C?H bonds (414kJmol−1), therefore, during cracking of alkanes, C?C bonds break more easily than C?H bonds.
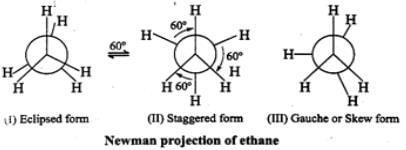
Q.23 Is it possible to isolate pure staggered ethane or pure eclipsed ethane at room temperature? Explain.
Answer: The energy difference between staggered and eclipsed forms of ethane is just 12.55kJmol−1 which is easily met by collisions of the molecules at room temperature. Therefore, it is not possible to isolate either pure staggered or pure eclipsed ethane at room temperature.
Q.24 trans-Pent-2-ene is polar while trans- but-2-ene is non-polar. Explain.
Answer: In trans-but-2-ene, the dipole moments of the two C−CH3bonds are equal and opposite and hence they exactly cancel out each other. Thus, trans-but- 2-ene is non-polar 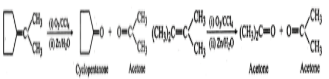 However, in trans-pent-2-ene, the+I-effect of CH3CH2-group is higher than that of CH3-group, therefore, the dipole moments of C−CH3 and C−CH2CH3 bonds are unequal. Although these two dipoles oppose each other, yet they do not exactly cancel out each other and hence trans-pent-2-ene has a small but finite dipole moment and thus is polar.
However, in trans-pent-2-ene, the+I-effect of CH3CH2-group is higher than that of CH3-group, therefore, the dipole moments of C−CH3 and C−CH2CH3 bonds are unequal. Although these two dipoles oppose each other, yet they do not exactly cancel out each other and hence trans-pent-2-ene has a small but finite dipole moment and thus is polar.
Q.25 Identify the organic products obtained in the following reaction: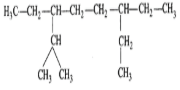
Answer: Dehydrohalogenation of the given alkyl halide can, in principle, yield two alkenes (I) and (II) 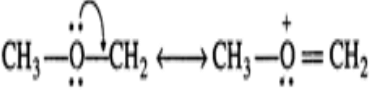 But according to Saytzeff rule, more highly substituted alkene, i.e., (I) being more stable is the major product of dehydrohalogenation. Therefore, in the above reaction, alkene (I) along with a small amount of alkene (II) is produced.
But according to Saytzeff rule, more highly substituted alkene, i.e., (I) being more stable is the major product of dehydrohalogenation. Therefore, in the above reaction, alkene (I) along with a small amount of alkene (II) is produced.
Q.26 Complete the following reactions with appropriate structures of products?
C6H5CH=CH2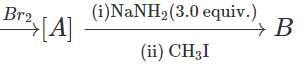
Answer: 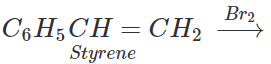
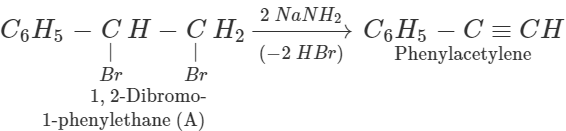
 Thus, [A] is 1, 2-dibromo 1-phenylethane and [B] is 1-phenylpropyne.
Thus, [A] is 1, 2-dibromo 1-phenylethane and [B] is 1-phenylpropyne.
Q.27 How will you prepare 3-methylbut-1-yne by starting with ethyne?
Answer: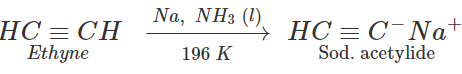

Q.28 Why HF forms H-bonding with ethyne even though it is non-polar in nature?
Answer: Due to sp-hybridization of carbon, the electrons of the C?H bond of ethyne are attracted towards carbon. As a result, carbon carries a partial negative charge while H carries a partial positive charge. Because of the presence of partial charge on H, ethyne forms H-bond with the F-atom of the HF molecule as shown: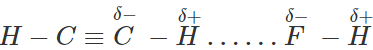
Q.29 Ethyne reacts with dil. H2SO4 in presence of mercury salts to give acetaldehyde but with dil. HCl under similar conditions, it gives vinyl chloride. Explain why?
Answer: First of all, mercury ions form a complex (I)with acetylene. Since, H2O is more nucleophilic than SO2−4 ion it attacks the complex (I) to form first vinyl alcohol which then tautomerises to given acetaldehyde 
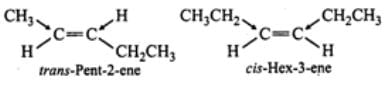
In case of dil. HCl, since, Cl− ion is more nucleophilic than H2O, it reacts with complex (I) to form vinyl chloride.
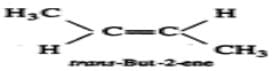
Q.30 How will you prepare (i) cis-pent-2-ene and trans-pent-2-ene by starting with ethyne?
Answer: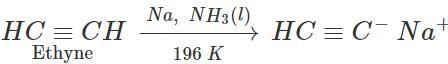
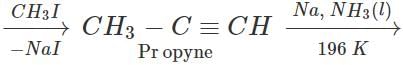
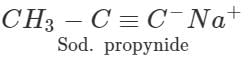
Q.31 Starting with ethyne, how will you prepare pentan-2-one?
Answer: Prepare pent-2-yne as discussed in Q. 13 above and then convert in to pentan-2-one by hydration.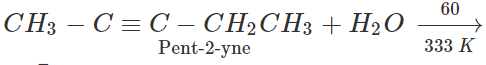
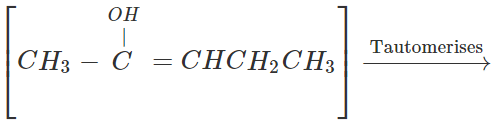
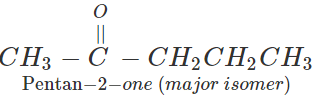
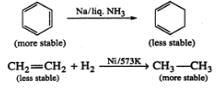
Q.32 How will you separate a mixture of ethane, ethylene and acetylene?
Answer: This mixture can be separated into its constituents by the following steps: Step 1. Pass the mixture of gases through Tollens' reagent when acetylene will form white precipitate of disilver acetylide while ethane and ethylene will pass through.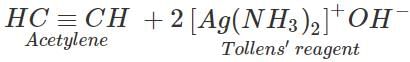
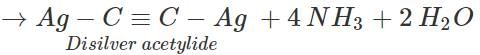
Separate the white ppt. by filtration and treat it with dil. HNO3 to regenerate acetylene. Collect it in a separate container.
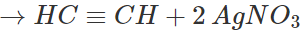
Step 2. Pass the remaining mixture of ethane and ethylene through cold conc. H2SO4when ethylene will be absorbed as ethyl hydrogen sulphate while ethane escapes. The ethane thus obtained is collected in a separate container. 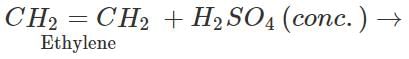
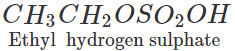
The ethyl hydrogen sulphate thus obtained is heated with conc. H2SO4 to 433 - 443 K when ethylene is obtained which is collected in a separate container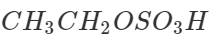
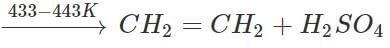
|
54 videos|125 docs|136 tests
|
FAQs on Short & Long Answer Questions: Hydrocarbons - Organic Chemistry for NEET
| 1. What are hydrocarbons? |  |
| 2. How are hydrocarbons formed? |  |
| 3. What are the different types of hydrocarbons? |  |
| 4. How are hydrocarbons used in everyday life? |  |
| 5. What are the environmental impacts of hydrocarbons? |  |

















