Class 10 Science Question Answers - Periodic Classification of Elements
Ques 1: What are the merits and demerits of Newland’s classification?
Ans: Merits:
(i) This classification worked well for lighter elements only up to Ca.
(ii) This classification gave us a relation between the properties of the elements and their atomic masses.
(iii) It was shown by this classification for the first time that there exists a periodicity in the properties of the elements.
Demerits:
(i) This classification failed when the heavier elements beyond Ca were arranged according to Newland’s law of octaves.
(ii) At the time of this law, noble gases were unknown. When noble gases were discovered, neon (Ne) between F and Na, and argon (Ar) between Cl and K, it becomes the ninth element and not the eighth which has the similar properties.
Ques 2: X and Y are two elements having which have similar properties and obey Newland’s law of octaves. How many elements are there in-between X and Y?
Ans: The law states that there are eight elements in an octave (row). No of elements between X and Y is six.
Ques 3: What is achievement of Dobereiner’s law of triads?
Ans: The law recognized the relation between atomic weight of an element and its chemical properties for the first time.
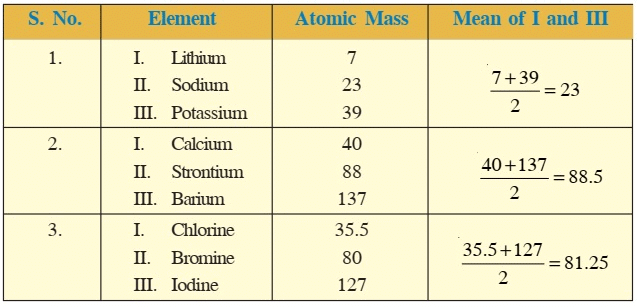
Ques 4: Lithium, Sodium and potassium were put in the same group on the basis of their similar properties.
i) What is the similarity in their properties?
Ans: i) Li, Na and K are very reactive alkali metals, which react with water resulting in the formation of an alkali and hydrogen gas.
Ques 5: State two reasons for rejecting law of octaves.
Ans: (i) The law did not extend properly beyond the element calcium.
(ii) The law did not provide any specific place for hydrogen.
Ques 6: Name four alkaline earth metals. To which group do they belong?
Ans: The four alkaline earth metals are beryllium, magnesium, calcium and strontium. They belong to II A group.
Ques 7: What is called diagonal relationship?
Ans: The similarity in properties between certain elements of different periods placed diagonally to one another in a periodic table is called diagonal relationship.
Ques 8: Define electron affinity.
Ans: It is defined as the amount of energy released in adding an extra electron to an isolated neutral gaseous atom in its lowest energy state, to convert it into gaseous ion. Electron affinity increases as one moves from left to right in periods or top to bottom in groups.
Ques 9: What is called electronegativity?
Ans: The property of atoms to attract electrons in a covalent bond is called electronegativity.
(i) In periods, electronegativity increases with the increase in atomic number.
(ii) In groups, electronegativity decreases with the increase in atomic number.
Ques 10: (a) Name an alkali metal other than lithium, sodium and potassium.
(b) Name an alkaline earth metals other than calcium, strontium and barium.
(c) Name one halogen other than chlorine, bromine and iodine.
(d) Name a non-metal having properties similar to carbon.
(e) Name a rare gas other than neon, krypton and xenon.
Solution: (a) Rubidium
(b) Magnesium
(c) Fluorine
(d) Silicon
(e) Argon.
Ques 11: Explain why the following statements are not correct?
i) All groups contain both metals and non-metals
ii) In group I, reactivity decreases with increases in atomic number
iii) Elements in a period become more metallic with increasing atomic number
iv) Atoms of elements in the same group have the same number of electrons.
Ans: i) All groups do not contain both metals and non-metals. Group I and II contain only metals.
ii) In group I, reactivity increases with increase in atomic number. This is because as the size of the atoms increases, the valence shell electrons can be easily removed.
iii) The metallic character decreases in a period with increase in atomic number. This is because across the period, the size of the atom decreases and the valence shell electrons are held more tightly.
iv) In a neutral atom the number of electrons is equal to the number of protons. In a group the atomic number increases as we move down.
Ques 12: Why are the elements sodium and chlorine in the same period of the Periodic table?
Ans: This is because the atoms of both the elements have three shells containing the electrons.
|
80 videos|359 docs|87 tests
|
FAQs on Class 10 Science Question Answers - Periodic Classification of Elements
| 1. What is the periodic classification of elements? |  |
| 2. How are elements classified in the periodic table? |  |
| 3. What are the advantages of the periodic classification of elements? |  |
| 4. How does the periodic classification of elements help in understanding chemical reactions? |  |
| 5. What are the main trends or patterns observed in the periodic table? |  |





















