Class 9 Science Chapter 2 Question Answers - Is Matter Around Us Pure?
| Table of contents |

|
| Very Short Answer Type Questions |

|
| Short Answer Type Questions |

|
| Long Answer Type Questions |

|
| Value-Based Questions |

|
Very Short Answer Type Questions
Q1. Define solvent.
The component of the solution that dissolves the other component in it is called the solvent.
Q2. Define solute.
The component of the solution that is dissolved in the solvent is called solute.

Q3. What is ‘tincture of iodine’?
A solution of iodine in alcohol is known as tincture of iodine. It has iodine (solid) as the solute and alcohol (liquid) as the solvent.
Q4. What are alloys?
The homogeneous mixture of two or more metals or a metal and non-metal is called an alloy.
Example: Steel is an alloy of Iron and Carbon.
Q5. Give one example of gas in liquid solution.
Cold-drinks, carbon dioxide gas as solute is mixed with water as a solvent.
Q6. How can a solution be dilute or concentrated?
The amount of solute dissolving in a solvent decides whether the solution is dilute or concentrated.
Q7. What is “concentration of a solution”?
The concentration of a solution is the amount of solute present in a given amount of solution or the amount of solute dissolved in a given mass or volume of solvent.
Q8. State the difference between aqueous and, non-aqueous solution.
Aqueous solutions have water as solvent and non-aqueous solutions do not haVe water as solvent.
Q9. What is “solubility” of a solute?
The amount of the solute present in the saturated solution at the given temperature is called its solubility.
Q10. What is a saturated solution?
The maximum amount of solute dissolved in a solvent at a given temperature is called saturated solution, where no more solute can dissolve further.
Q11. What is an unsaturated solution?
If the amount of solute contained in a solution is less than the saturation level, it is called an unsaturated solution.
Q12. How can you convert the saturated solution into unsaturated or vice-versa?
Saturated solution on heating becomes unsaturated and unsaturated solution on cooling becomes saturated.
Q13. Why water is called universal solvent?
Water can dissolve large number of substances in it.
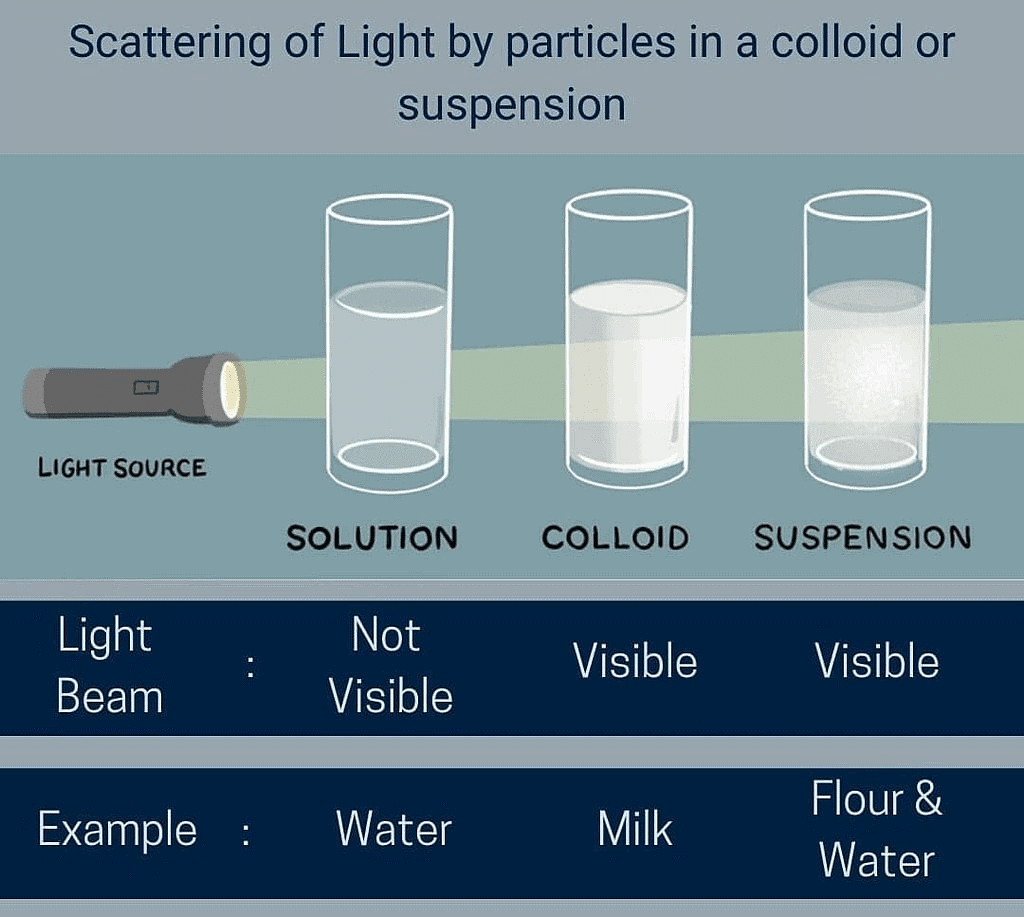
Q14. What is Tyndall effect?
The scattering of light by colloidal particles is known as Tyndall effect.
Q15. How can we separate colloidal mixtures?
By centrifugation, in a centrifuge machine, the colloidal solution is kept in a test tube, rotated very fast and due to centrifugal force the colloidal particles are separated.
Q16. What is an emulsion?
When both the dispersed phase and dispersing medium is liquid, it is called emulsion.
Example: Milk, Face Cream.
Q17. What is aerosol?
When the solid or liquid is dispersed in a gas it is called aerosol.
Example: Smoke, Fog.
Q18. What is the principle for separation of immiscible liquids?
The principle of separating immiscible liquids into layers depending on their densities. The less denser liquid collects at the top and more denser liquid at the bottom.
Q19. What is chromatography?
Chromatography is the technique used for the separation of those solutes that dissolve in the same solvent.
Q20. What is distillation?
Distillation is the separation technique of two miscible liquids that boils without decomposition and have sufficient difference in their boiling points.
Q21. How can you separate two liquids that have less than 25 K difference of boiling points?
To separate a mixture of two or more miscible liquids for which the difference in boiling points is less than 25 K, is fractional distillation.
Q22. What is condenser?
It is an apparatus used to convert gas into liquid by cooling it.
Q23. What is crystallisation?
When a saturated solution is heated and allowed to cool slowly, crystal of the solute dissolved in the saturated solution is separated from it. It is used to purify solids.
Short Answer Type Questions
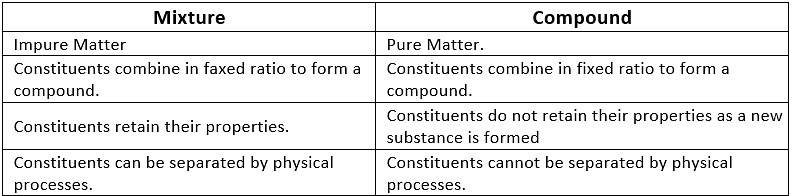
Q1. Why is mixture called an impure substance?
Mixture consists of different components which retain their properties and can be easily separated by physical processes, hence it is called as impure substance.
Q2. Give the differences between mixture and compound.
Q3. Distinguish between a physical change and chemical change.
Q4. State the properties of a solution.
Properties of a solution are:
- A solution is a homogeneous mixture.
- Particles of a solution are smaller than 1 nm and cannot be seen by naked eyes.
- Do not scatter a beam of light.
- Solute particles cannot be separated from the mixture by the process of filtration and thus, solution is stable.
Q5. State the properties of a suspension.
Properties of a suspension:
- Suspension is a heterogeneous mixture having particle size greater than 100 nm.
- The particles of a suspension can be seen by naked eyes.
- Particles can scatter a beam of light.
- It is unstable.
Q6. What is a colloidal solution?
It is a heterogeneous solution which appears to be homogeneous, particles size is very small and so cannot be seen with naked eyes but it is stable.
Example: Milk and Blood.
Q7. State the properties of colloidal solution.
Properties of colloidal solution:
- It is a heterogeneous mixture having particle size between 1 nm to 100 nm.
- The size of particles is very small, cannot be seen with naked eyes.
- It scatters a beam of light.
- They are stable as the particles do not settle when left undisturbed.
Q8. Give the applications of centrifugation.
Application of centrifugation are:
- Used in diagnostic laboratories for blood and urine test.
- Used in dairies and home to separate butter from cream.
- Used in washing machines to squeeze out water from wet clothes.
Q9. Give the applications of chromatography.
Applications of chromatography are:
- To separate colours in a dye.
- To separate pigments from natural colours.
- To separate drugs from the blood.
Q10. Why is crystallisation better than evaporation?
Crystallisation is a process that separates a pure solid in the form of its crystals from a solution.
Crystallisation is better than evaporation because during Evaporation:
- Some solids decompose or some, like sugar, may get charred on heating to dryness.
- Some impurities may remain dissolved in the solution even after filtration which on evaporation contaminates the solid.
Q11. How will you separate a mixture of oil and water?
- To separate a mixture of oil and water, we need a separating funnel as both are immiscible liquids. Pour the mixture into the separating funnel and let the funnel stand undisturbed for some time.
- So that separate layer of oil and water are formed. Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
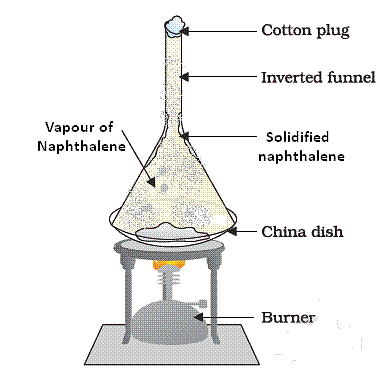
Q12. A student is given a mixture of naphthalene ball’s powder and common salt. He needs to separate this mixture. How will he do this?
- The properties of both naphthalene and common salt should be known before we choose the separation technique.
- Naphthalene is a sublimate which on heating changes to gaseous state directly. Hence to separate a volatile compound (sublimate) from a non-volatile compound (non-sublimate), the sublimation process is used.
Sublimation of Naphthalene- In a China dish, the mixture is kept and is placed on a stand. An inverted funnel is kept over the mixture in China dish with plugged stem. The sublimate on heating gets collected on the funnel and common salt remains in the China dish.
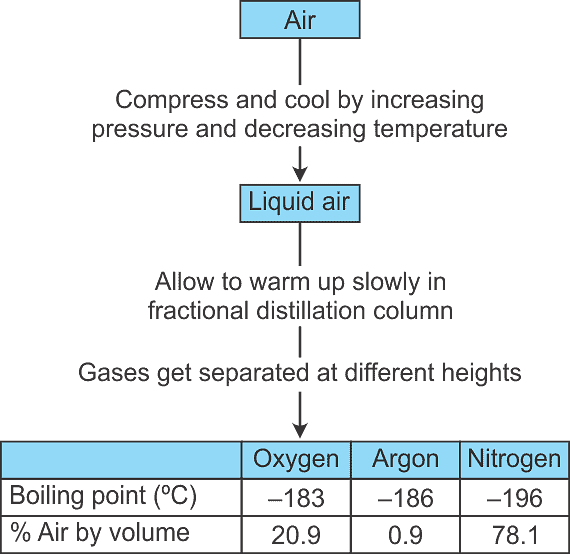
Q13. How can we obtain different gases from air?
Air is a homogeneous mixture and its components can be separated by fractional distillation.
The flow diagram shows the steps involved in the process.
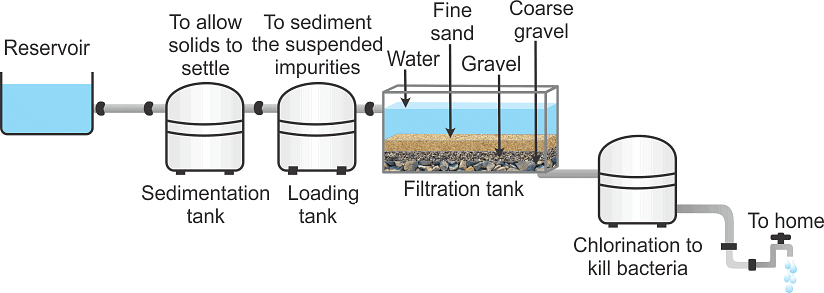
Q14. Draw a flow diagram to show the water purification system in waterworks.
- Water is collected in the reservoir → The water is sent to sedimentation tank to allow solids to settle → It is then passed to 2nd tank i.e., loading tank, so that the suspended impurities are loaded to settle down as sediment. → Then water reaches the filtration tank
- In filtration, tank water passes through different layers of sand and gravel as shown in the above figure this is for adsorption of impurities.
The clear water reaches a chlorinated tank where water is mixed with bleaching powder/chlorine to kill bacteria and then supplied to houses.
Q15. Why is air considered as a mixture and not a compound?
Air is considered as a mixture because it exhibits following properties:
- Each component present in air retains its properties.
- Each component can be separated by simple physical processes.
- The components do not have any fixed proportion. All gases are present in different amount.
Example: In greener area—more oxygen and water vapour is present; near industrial area—air consists of a lot of impurities and smoke suspended in it.
Q16. How can you prove that water is a compound?
Water is a compound because if we pass electricity through it then at two different electrodes, we get two different gases i.e., oxygen and hydrogen during the electrolysis of water. The ratio of oxygen: hydrogen is 1: 2 by number of molecules.
(i) The properties of oxygen and hydrogen gases sire entirely different from that of liquid water.
(ii) The ratio of oxygen: hydrogen combination is always constant i.e., 1: 2 by volume.
(iii) To separate the components of water, we need electrolytic cell, and it is not a simple process.
Q17. How can we convert saturated solution into unsaturated by heating?
- A saturated solution is said to be saturated at a given temperature when there is no more scope of solute particles to dissolve /dissociate into water. It is because the solute particle has taken all the intermolecular space present in the solvent.
- On heating, the molecules of solvent gain kinetic energy, start vibrating and try to move away from each other thereby accommodating some more solute particle in this space and hence it becomes an unsaturated solution.
Q18. What is the difference in fog and smoke?
Fog is a colloidal solution with liquid dispersed in gas.
Smoke is a colloidal solution with solid dispersed in gas.
Q19: If 20g of salt is present is 220 g of solution, calculate the concentration of
Concentration of solution = Mass of solute/(Mass of solute = Mass of solvent) × 100
Mass Solute = 20g
Mass of solute + solvent = 220g
∴ Concentration of solution = 20/220 × 100 = 9.09%
Long Answer Type Questions
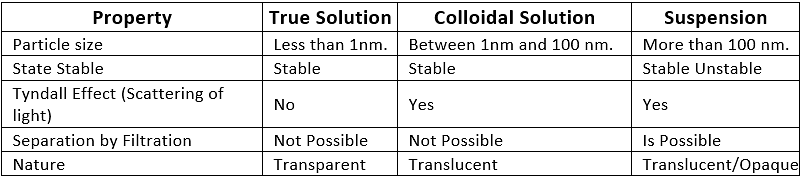
Q1. Give the difference between true solution, colloidal solution and suspension.
The difference between true solution, colloidal solution and suspension
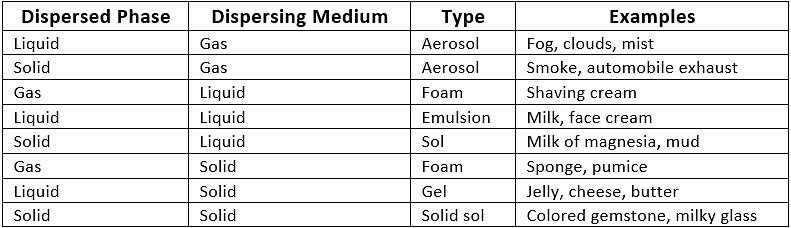
Q2. State the different types of colloids with examples.
Q3. (a) Define solution.
(b) Give different types of solutions with one example each.
(a) Solution: It is a homogeneous mixture of two or more substances. It consists of solute and solvent.
(b) Different types of solution:
- Based on solvent—Aqueous and non-aqueous Aqueous solution has water as solvent (sugar + water) Non-aqueous solution has some other solvent but not water.
Example: (sulphur + carbon disulphide)- Depending on the amount of solute dissolved in solvent—Dilute solution and concentrated solution.
- Dilute solution—Less amount of solute particles are present in a solvent.
Concentrated solution—Amount of solute present in its maximum capacity in a solvent.- Amount of solute present in its maximum capacity at a given temperature—Saturated and unsaturated solution.
- Saturated Solution—It is a solution in which no more solute can further dissolve in a given solvent at a given temperature.
- Unsaturated Solution—It is a solution in which some more solute can dissolve in a solvent at a given temperature.
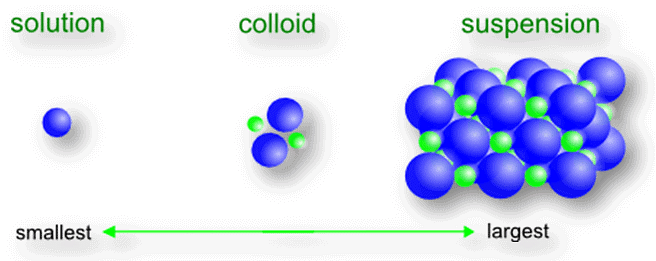
- Depending on the size of solute particles:
(i)True solution Size is very small and particles cannot be seen through naked eyes
(ii) Suspension Size is very big and can be seen through naked eyes
(ii) Colloid Size is intermediate between true solution and suspension
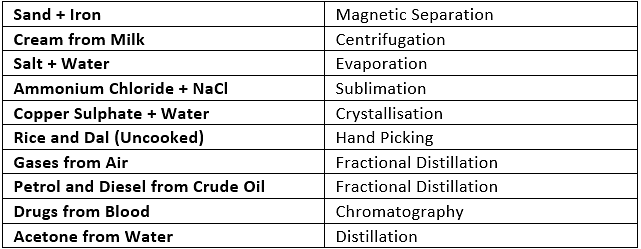
Q4. How can you separate the following mixtures?
(a) Sand + iron
(b)Cream from milk
(c) Salt + water
(d) Ammonium chloride + NaCl
(e) Copper sulphate + water
(f) Rice and dal (uncooked)
(g) Gases from air
(h) Petrol and diesel from crude oil
(i) Drugs from blood
(j) Acetone from water
Value-Based Questions
Q1. Anil’s sister accidentally added some water into the bottle containing olive oil and she was afraid of the scolding. Anil helped his sister and separated the water from olive oil using bottle as a separating funnel.
(a) What is the principle of using and working of separating funnel?
(b) Suggest two separation techniques used to separate liquid mixtures.
(c) What value of Anil is seen in the above case?
(a) The principle of separating funnel is difference in the densities of two liquids.
(b) Liquid mixtures can be separated by distillation and fractional distillation.
(c) Anil showed the value of helping, caring and responsible behaviour.
Q2. Preeti saw a labour entering into the sewage manhole immediately after removing the lid. She promptly stopped the labour from entering into the manhole and told him to wait for some time before he enters into it.
(a) What will happen if the labour immediately enters into the manhole for cleaning) after removing the lid?
(b) Name main gases that are released from the manhole.
(c) What value of Preeti is seen in the above act?
(a) If the labour immediately enters the manhole on removing its lid he would die due to suffocation and inhalation of poisonous gases which are compressed and released by sewage.
(b) Gases released from the sewage manhole are methane, carbon dioxide and hydrogen sulphide.
(c) Preeti shows the value of moral responsible behaviour and aware citizen.
Q3. Prasanna wanted to buy a deodorant from the shop. While buying a bottle he felt that it was slightly heavier than usual deodorant bottle that he purchased everytime. He read the weight mentioned on the bottle and told the shopkeeper to weigh the same. He found the bottle was heavy and on opening the deodorant bottle he found it half-filled with water. He complained the matter to the consumer authority.
(a) Define density.
(b) Apart from water what is the other substance that some shopkeepers add into the deodorant.
(c) What value of Prasanna is reflected in this act?
(a) Density of any substance is defined to be the mass of the substance per unit volume.
(b) One can add some cheap gases or compressed air in the deodorant bottles.
(c) Prasanna showed the value of being having leadership quality, rightful, aware and responsible citizen.
Q4. Rita’s father always got his vehicle checked for pollution control. He got it tested for the aerosol if released by his car. He also uses unleaded petrol and makes use of public transport wherever possible. He sparingly use his car.
(a) What is aerosol?
(b) What happens when smoke released from vehicle mixes with fog?
(c) What are the values of Rita’s father is reflected here?
(a) When the solid or liquid is dispersed in a gas it is called aerosol.
Example: Smoke.
(b) When smoke mixes with fog it forms smog.
(c) Rita’s father is an aware citizen, environmentally concerned and dutiful.
|
88 videos|369 docs|67 tests
|
FAQs on Class 9 Science Chapter 2 Question Answers - Is Matter Around Us Pure?
| 1. What is Matter Around Us Pure? |  |
| 2. What are pure substances? |  |
| 3. What are mixtures? |  |
| 4. What are some methods to separate mixtures? |  |
| 5. Why is it important to study Matter Around Us Pure? |  |

|
Explore Courses for Class 9 exam
|

|