Solved Practice Questions: Conformation of acyclic systems: Substituted ethane/ n-propane/ n-butane | Organic Chemistry PDF Download
Q.1. Write all the important conformations of n-butane.
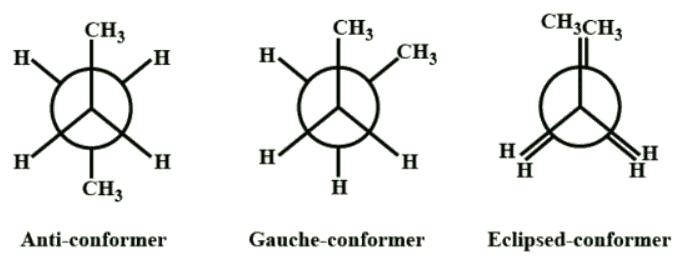
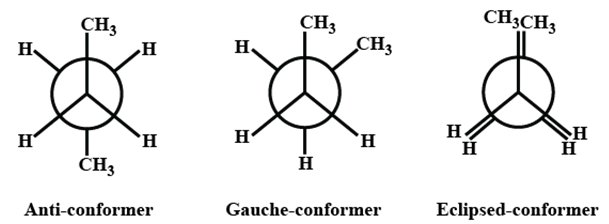
Conformations of butane is as follows:
- Anti-conformer
- Gauche
- Eclipsed
Q.2. In the following the most stable conformation of n−butane is:
(a) 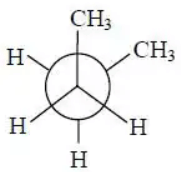
(b) 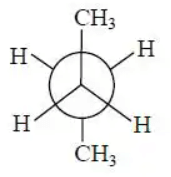
(c) 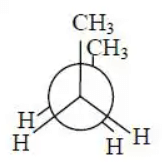
(d) 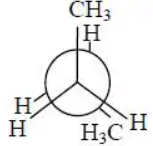
Correct Answer is Option (b)
The anti staggered conformation of n-butane is more stable than gauche-staggered and eclipsed conformations of n-butane. In anti staggered n-butane, the methyl groups are placed at a dihedral angle of 180°, and the steric hindrance is minimal in anti-form than in gauche form.
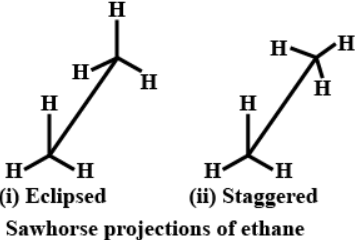
Q.3. Draw Sawhorse projection for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Stability of conformation: In staggered form of ethane , the electron clouds of carbon-hydrogen bonds are as far apart as possible. Thus , there are minimum repulsive forces, minimum energy and maximum stability of the molecule. On the other hand, when the staggered form changes into the eclipsed form, the electron clouds of the carbon-hydrogen bonds come closer to each other resulting in increase in electron cloud repulsion. To check the increased repulsive forces, molecule will have to posses more energy and thus has lesser stability. Staggered form is more stable.
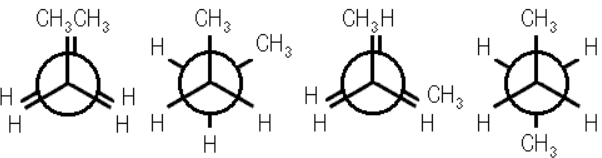
Q.4. Why propane has only one eclipsed conformation while butane has two? Explain.
For purpose of writing conformation, propane may be regarded as 1− methyl methane while butane may be regarded has 1,2− dimethylethane. Since propane has only one methyl group, therefore it has only one eclipsed conformation in which there is one strong methyl-hydrogen interaction and two weak hydrogen-hydrogen interactions.
However, in the case of butane there two methyl groups, one on each carbon and hence has two eclipsed conformations. (i) fully eclipsed conformation in which there are one severe methyl-methyl interaction and two weak hydrogen interaction. (ii) partially eclipsed conformation in which there are two strong methyl-hydrogen interactions and one weak hydrogen-hydrogen interaction.
For Newman Projections of these conformations refer.
Q.5. The conformations of n-butane, commonly known as eclipsed, gauche and anti-conformations can be interconverted by:
(a) rotation around C − H bond of a methyl group
(b) rotation around C − H bond of a methylene group
(c) rotation around C1 − C2 linkage
(d) rotation around C2 − C3 linkage
Correct Answer is Option (d)
Rotation around C2 − C3 linkage
|
39 videos|92 docs|46 tests
|