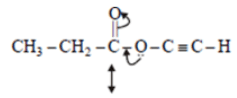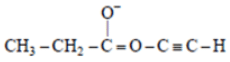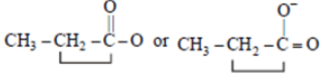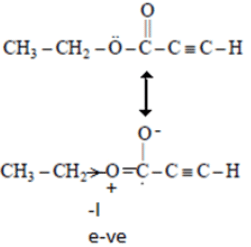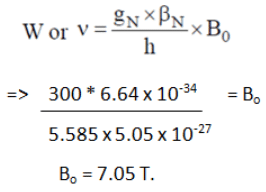Solved Practice Questions on Mass Spectroscopy | Organic Chemistry PDF Download
Q.1. Which type of ionic species are allowed to pass through the slit and reach the collecting plate?
(a) Negative ions of all masses
(b) positive ions of the specific mass
(c) Negative ions of the specific mass
(d) Positive ions of all masses
Correct Answer is Option (b)
Positive ions of specific mass pass through the slit and reach the collecting plate. The ion currents are measured using sensitive electrometer tube. The ions reaching the collecting plate are measured.
Q.2. The spectrum of a compound with molecular formula C5H602 is shown below. IR spectrum shows medium intensity band at 3270 and 2180 cm-1. What will be the structure of compound? Chemical reference: 1.3 (3H, t); 2.8 (1H, s), 4.3 (2H, q).
(a) 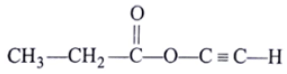
(b) 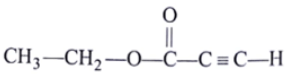
(c) 
(d) 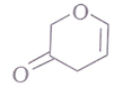
Correct Answer is Option (b)
We have –>C5H6O2
IR data: 3270 cm–1, 2180 cm–1 shows it can be an alkyne
NMR data: 1.3 (3H, t); 2.8 (1H, s); 4.3 (2H, q)
Now out of (a) & (b)
(a)
So, no such the electronegative difference between both cases C-C Bond.
(b)So, electronegative difference between both cases C-C Bond is high. Hence 2H peak will at 4.3.
Q.3. Which species of the following is used to bombard with the sample for which mass spectroscopy has been performed?
(a) Alpha particles
(b) Neutrons
(c) Electrons
(d) Protons
Correct Answer is Option (c)
In the mass spectrometer, the sample which is to be analysed is bombarded with electrons, which leads to the formation of the ions. The ions are sorted out by accelerating them through electric and magnetic field. A record of the number of different kinds of ions is called mass spectrum.
Q.4. What are the main criteria on which mass spectrometer used for?
(a) Composition in sample
(b) Relative mass of atoms
(c) Concentration of elements in the sample
(d) Properties of sample
Correct Answer is Option (b)
A mass spectrometer generates multiple ions from the sample under investigation, it then separates them according to their specific mass-to-charge ratio (m/z) or we can say on relative mass of atoms, and then records the relative abundance of each ion type.
Q.5. In which state of matter mass spectroscopy is being performed?
(a) solid
(b) liquid
(c) gaseous
(d) plasma
Correct Answer is Option (c)
In the mass spectrometric analysis of compounds is firstly done by production of gas phase ions of the compound, basically by electron ionization. This molecular ion undergoes fragmentation.
Q.6. Which of the following main component of mass spectroscopy deal with resolving the ions into their characteristics mass components according to their mass-to-charge ratio?
(a) Ion Source
(b) Analyzer
(c) Detector System
(d) Analyzer tube
Correct Answer is Option (b)
The complete process involves the conversion of the sample into gaseous ions, with or without fragmentation, which is then characterized by their mass to charge ratios (m/z) by an analyser. Eventually, the second analyzer will analyse fragment the selected ions and detect the ions emerging from the last analyzer and measure their and relative abundances with the detector that converts the ions into electrical signals.
Q.7. Separation of ions in mass spectrometer take place on the basis of which of the following?
(a) Mass
(b) Charge
(c) Molecular weight
(d) Mass to charge ratio
Correct Answer is Option (d)
Mass spectrometer separates ions on the basis of mass to charge ratio i.e. m/z. In the spectrum of a pure compound, the molecular ion, if present, appears at the highest value of m/z (followed by ions containing heavier isotopes) and gives the molecular mass of the compound. Most of the ions are singly charged. Hence, the mass to charge ratio is equal to the mass.
Q.8. An organic compound Q exhibited the following spectral data obtained by mass spectroscopy.
IR: 1760 cm–1
1HNMR: chemical reference (ppm): 7.2 (IH, d, 16.0 Hz), 5.1 (IH, m), 2.1 (3H, s), 1.8 (3H, d, J = 7.0 Hz)
13CNMR chemical reference (ppm): 170 (carbonyl carbon). What is compound Q?
(a) 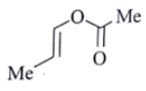
(b) 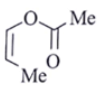
(c) 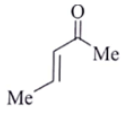
(d) 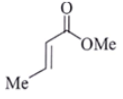
Correct Answer is Option (a)
IR: 1760 cm–1
Some options are not possible because of IR data.
1760 cm–1 –> Represent either ester or anhydride
ester –>with cross conjugation or anhydride
chemical reference: 7.2 (1H), d, J = 16.0 Hz
This ‘J’ shows it is a trans alkene.
Q.9. A compound of molecular formula C8H7ClO shows a prominent band in its IR spectrum at 1690 cm-1. 1H NMR spectrum revealed only two major types of protons in the ratio of 5: 2. Which one of the following structures best fits the above data?
(a) 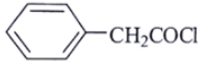
(b) 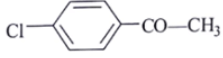
(c) 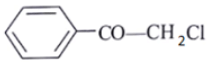
(d) 
Correct Answer is Option (a)
C8H7ClOIR data: 1690 cm–1
NMR data: two types of protons in 5: 2 ratio. Only two types of protons.
Ketone with conjugation shows peaks at 1690 cm–1.
Q.10. A PMR spectrometer operates at 300 MHz. Find the value of magnetic field.
Given: gN = 5.585 and BN = 5.05 x 10-27 JT-I.
(a) 7.05 T
(b) 6.38 T
(c) 7.58 T
(d) 5.93 T
Correct Answer is Option (a)
According this formula of frequency:
Q.11. Who discovered the mass spectrometer?
(a) Francis Aston
(b) J. J Thomson
(c) Ernest O. Lawrence
(d) Walter Kaufmann
Correct Answer is Option (b)
The mass spectrometer was invented by JJ THOMSON. He performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube. Further modified by F. W. Aston shortly after World War I. He constructs the first velocity focusing mass spectrograph which has mass resolving power of 130.
Q.12. Which of the following statement is false for mass spectroscopy?
(a) Mass spectroscopy is used to identify unknown compounds within a sample, and to elucidate the structure and chemical properties of different molecules
(b) Particle are characterized by their mass to charge ratios (m/z) and relative abundances
(c) This technique basically studies the effect of ionizing energy on molecules
(d) This technique can be used on all state of matter
Correct Answer is Option (d)
A mass spectrum measures the masses within a sample. Particles are characterized by their mass to charge ratios (m/z) and relative abundances. This technique basically studies the effect of ionizing energy on molecules and is applied to pure samples as well as complex mixtures. This technique is only possible to perform in the gaseous state.
|
39 videos|92 docs|46 tests
|