Matter in Our Surroundings: Nature & Characteristics of Particles of Matter | Science Class 9 PDF Download
| Table of contents |

|
| What is Matter? |

|
| Physical Nature of Matter |

|
| Characteristics of Particles of Matter |

|
| Classification of Matter |

|
| Activity |

|
What is Matter?
Anything that occupies space and has mass is called matter. In other words, they have both mass and volum
Physical Nature of Matter
1. Matter is made up of Particles
The particle nature of matter can be demonstrated by a simple activity.
- Experiment:
(i) Take about 50 ml water in a 100 ml beaker.
(ii) Mark the level of water.
(iii) Add some salt to the beaker and stir with the help of a glass rod.
(iv) Observe the change in water level.
- Observation:
(i) It is observed that the crystals of salt disappear.
(ii) The level of water remains unchanged. - Explanation:
A water molecule consists of hydrogen and oxygen atom, between hydrogen and oxygen, there are large empty spaces. These empty spaces are known as void.
Void: When we add salt to the water, it goes into that void. As a result, we do not see any change in volume.
- Conclusion:
This activity shows that matters are made of small particles. And there is space between these particles.
2. How Small are these Particles of Matter?
- Experiment:
(i) Take a 250 ml beaker and add 100 ml water to it.
(ii) Now add 2-3 crystals of potassium permanganate (KMnO4) and stir with a glass rod in order to dissolve the crystals.
(iii) Take 10 ml of this solution and add to 100 ml of water taken in another beaker.
(iv) Take 10 ml of this diluted solution and put into 100 ml of water taken in still another beaker.
(v) Repeat this process 10 times observe the colour of the solution in the last beaker. Diffusion of Potassium Permanganate in Water
Diffusion of Potassium Permanganate in Water - Observation:
(i) When we add potassium permanganate in water, the colour of water changes to pink.
(ii) Dilution decreases the colour intensity of the solution. - Explanation:
(i) A small amount of Potassium permanganate contain millions of its molecules. When we dissolve potassium permanganate in water, its molecule spread uniformly in the solution and give a pink appearance.
(ii) Dilution lowers the amount of the particles in a subsequent solution. As a result, we see a lower colour intensity. - Conclusion:
This activity proves that matter is made up of tiny particles.
Characteristics of Particles of Matter
1. Particles of Matter are Continuously Moving
- If an incense stick (Agarbatti) is lighted and placed in one corner of a room, its pleasant smell spreads in the whole room quickly.
 Agarbatti
Agarbatti - It demonstrates that the particles of matter possess motion. When we light an incense stick, it produces some gases (vapour) having a pleasant smell.
- The particles of these gases due to motion spread in the entire room. As a result, we can observe the smell of the lighted incense stick from a long distance.
- This shows that Matters consist of small particles which are moving continuously. This means that particles of matter possess kinetic energy.
Activity
- Aim:
To demonstrate that the Kinetic Energy of particles increases with an increase in temperature. - Experiment:
(i) Take two beakers. To one beaker add 100 ml of cold water and to the other beaker add 100 ml of hot water.
(ii) Now add some crystals of potassium permanganate or copper sulphate to both the beakers.
Kinetic energy: Kinetic energy of an object is the measure of the work an object can do by virtue of its motion. The kinetic energy is 1/2 mv2. To accelerate an object, we have to apply force. To apply force, we need to do work. When work is done on an object, energy is transferred, and the object moves with a new constant speed. We call the energy that is transferred kinetic energy, and it depends on the mass and speed achieved.
- Observation:
It is observed that crystals in hot water diffuse and dissolves faster than in a beaker containing cold water. - Conclusion:
(i) All substances have some kinetic energy in it. When we heat a substance, its kinetic energy increases.
(ii) Heating water results in an increase in its kinetic energy; as a result, we see that crystals dissolve in much lesser time.
(iii) From these activities, it is observed that when two different forms of matter are brought into contact, they intermix spontaneously.
(iv) This intermixing is possible due to the motion of the particles of matter and also due to the spaces between them.
Diffusion
- This spontaneous intermixing of particles of two different types of matter is called diffusion.
- The rate of diffusion becomes faster with an increase in temperature because, at a higher temperature, the particles have more energy and hence move faster.
 Diffusion is a natural phenomenon and never stops
Diffusion is a natural phenomenon and never stops
Examples:
- In our daily life, we make use of diffusion in many places. We add sugar in water to make tea or juices; here, the sugar molecule diffuses into the water through diffusion.
- A plant produces oxygen; this oxygen diffuse into the air and we get oxygen for breathing.
- The smell of food being cooked in the kitchen reaches us even from a considerable distance.
- When someone opens a bottle of perfume in one corner of a room, its smell spreads in the whole room quickly.
- The leakage of cooking gas (LPG) in our homes is detected due to the diffusion of a strong-smelling substance present in the cooking gas, into the air.
2. Particles of Matter Attract each other
- There are some forces of attraction between the particles of matter which bind them together. The force of attraction between the particles of the same substance is known as cohesion.
- The force of attraction (or cohesion) is different in the particles of different kinds of matter. In general, the force of attraction is maximum in the particles of solid matter and minimum in the particles of gaseous matter.
Activity
- Aim:
To demonstrate the attractive forces between particles of matter. - Experiment:
(i) Take a piece of iron wire, a piece of chalk and a rubber band.
(ii) Try to break them by hammering, cutting or stretching. - Observation:
(i) Hammering a piece of the iron nail does not break the nail but flatten its surface.
(ii) Hammering chalk breaks the chalk and gives us powdered chalk.
(iii) We can stretch the rubber band to a large length without any break. - Conclusion:
(i) Since energy is required to break crystals of matter into particles.
(ii) It indicates that particles in the matter are held by some attractive forces; the strength of these attractive forces varies from one matter to another.
3. Particles of Matter have Space between Them
- There are small voids between every particle in a matter.
- This characteristic is the concept behind the solubility of a substance in other substances.
Activity
- Aim:
To demonstrate the space between particles of matter. - Experiment:
(i) Take a glass of water.
(ii) Put a teaspoon of salt/sugar and mix them properly. - Observation:
The water is still clear. - Explanation:
This is because the particles of salt/sugar get into the interparticle spaces between the water particles. - Conclusion:
(i) This proves that there are voids between particles of a substance.
(ii) If you add more salt/sugar, it will dissolve until all the space between water particles get filled.
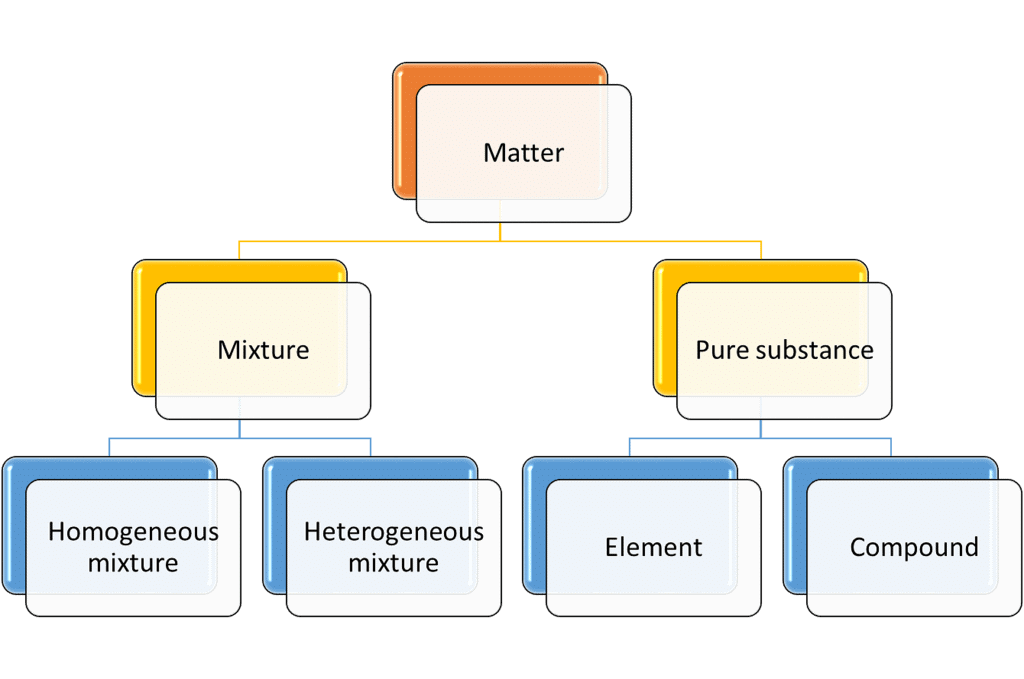
Classification of Matter
- The matter can be classified into different categories depending upon its physical or chemical nature.
- On the basis of chemical nature, the matter is classified as:
1. Element
2. Compound
3. Mixture - Elements and compounds are pure substances, whereas a mixture contains two or more pure substances.
Note:
Material: The term used to describe a particular kind of matter is called material.
Examples: Wood, Water and Marble.
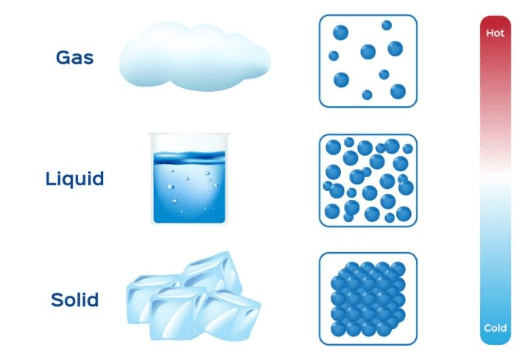
- On the basis of the physical state, the matter is categorized as:
1. Solid
2. Liquid
3. Gas
Example: Air, Water and ice. - Changes of state are also matters of everyday experience.
Example: Ice melts and water freezes, water changes into steam on heating and steam condenses to liquid water on cooling.
Solid State
- A solid possesses a fixed volume and a definite shape, distinct boundaries and a definite mass.
- Examples of solids:
(i) A wooden block should be called a solid.
Explanation: A wooden block has a fixed shape and is rigid. Hence, it should be called solid.
(ii) A rubber band undergoes a change in shape on stretching. Still, we call it a solid because it regains the same shape when the force is removed. - Solids are rigid and almost incompressible.
- Solids may break under force, but it is difficult to change their shape.
- Solids generally possess high densities.
- Solids do not exhibit diffusion.
- In solids, intermolecular forces of attraction are stronger.
Examples: Table, Chair, Common Salt, Silver, Ice, Diamond, Stone, Sugar etc.
Note:
- Solids generally do not exhibit diffusion due to smaller inter-particle spaces and the absence of translatory motion.
- Some examples in which solids show diffusion:
(i) If we write something on a blackboard and leave it uncleaned for a considerable period of time (say, at least 10 to 15 days), we will find that it becomes quite difficult to clean the blackboard afterwards. This is due to the fact that some of the particles of chalk have diffused into the surface of backboard.
(ii) If two metal blocks are bound together tightly and kept undisturbed for a few years, then the particles of one metal are found to have diffused into the other metal.
Liquid State
- A liquid possesses a definite volume, a definite mass, but no definite shape.
- Examples: Water, Alcohol, Milk, Diesel, Petrol, Kerosene Oil, Vegetable Oil, Fruit Juices etc.
- Liquids are also almost incompressible but are not rigid. In fact, they can flow from a higher to a lower level.
- Liquids have a property of fluidity and acquire the shape of the container in which they are kept.
- Liquids can undergo diffusion.
- Liquids also have high densities but less than solids.
- In liquids, intermolecular forces of attraction are weaker than solid.
- Water molecules in a container are in continuous motion. As a result, they do not have their own shape and flow easily.
- Solids, liquids as well as gases can diffuse into liquids. This is due to the fact that the interparticle spaces in liquids are larger and the particles in the liquid state move freely.
Gaseous State
- The matter in a gaseous state has neither a definite volume nor a definite shape, but it has a definite mass.
- It acquires the shape and volume of the container.
- Gases are highly compressible.
 Evaporation an Example of Gaseous Matter
Evaporation an Example of Gaseous Matter
Activity
- Aim:
To demonstrate air is compressible while water and solid are not, activity wants us to fill three syringes, each with one type of object viz, water, chalk powder, and air and ask us to compress it. Experimental Setup
Experimental Setup - Observation:
With some force, we are able to compress the syringe filled with air while other syringes are completely non-compressible. - Explanation:
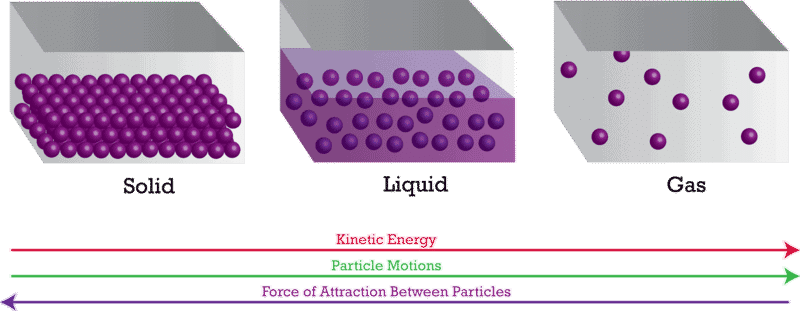
(i) Solid, liquid and gases have a different amount of spaces between their constituent molecules.
(ii) A solid has the least space between the molecule which does not move; while gases have the largest intermolecular spaces.
(iii) When we compress a gas, its intermolecular space decreases. Since gases have large intermolecular space, we are easily able to compress them with force.
(iv) While, in the case of liquid and solid has small intermolecular space makes it completely impossible to compress.
(v) In the figure below, a, b and c show the magnified schematic pictures of the three states of matter. The motion of the particles can be seen and compared in the three states of matter.
- Conclusion:
This experiment demonstrates that gases are compressible while liquids and solids are not.
Table: Comparison of Characteristic Properties of Solids, Liquids and Gases

For further topics in the lesson, check out this link - Changes in States of matter and Evaporation.
|
88 videos|369 docs|67 tests
|
FAQs on Matter in Our Surroundings: Nature & Characteristics of Particles of Matter - Science Class 9
| 1. What is matter? |  |
| 2. What are the characteristics of particles of matter? |  |
| 3. What are the different classifications of matter? |  |
| 4. How does the physical nature of matter affect its behavior? |  |
| 5. What is the nature of particles of matter in our surroundings? |  |

|
Explore Courses for Class 9 exam
|

|
























