Stereo: D/L, R/S , Erythro/Threo Nomenclature of Organic Compounds | Organic Chemistry PDF Download
D,L System of Nomenclature
D,L system of Nomenclature is the most commonly used system for assigning the configuration to be given enantiomer. The D,L system is often used to classify molecules that are chiral (i.e., molecules that have a non-superimposable mirror image).
- D (Dextrorotatory): Molecules that rotate plane-polarized light to the right.
- L (Levorotatory): Molecules that rotate plane-polarized light to the left.
- The oldest system of nomenclature of enantiomers is D,L system.
- In this system, the configuration of all the compounds was designated concerning glyceraldehydes the configuration of which was taken as an arbitrary standard by Rosanoff (1906).
- (+) Glyceraldehyde having the OH group on the right and hydrogen on the left-CHO and-CH2OH group being on the top and the bottom respectively was arbitrarily given the configurational symbol D.
- The mirror image compound of (+) Glyceraldehyde was given the configuration L.
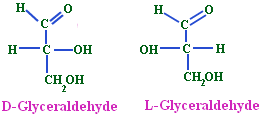
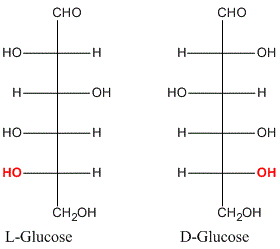
- Any compound that can be prepared from or converted into D (+) Glyceraldehyde will belong to D-series and the same is true for L-(-)-Glyceraldehyde.
- There is no change in configuration if a reaction doesn’t involve the cleavage of a bond to the chiral centre.
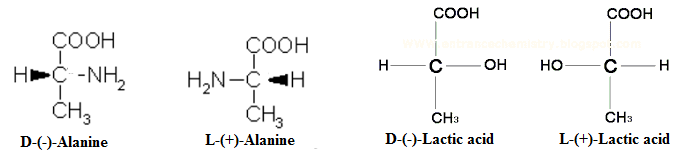
Drawbacks of D, L Nomenclature
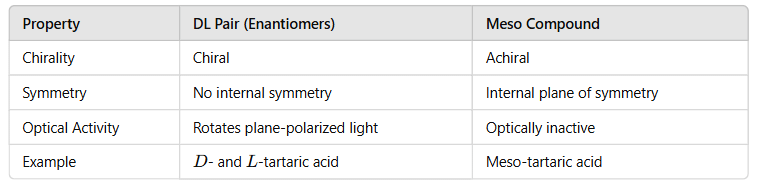
- It indicated nothing more than relative configurations i.e. there was no way of deciding whether these stereochemical representations reflected reality.
- D, L –nomenclature creates confusion in assigning the configurations to some compounds. Tartaric acid may be assigned L configuration with respect to the bottom COOH and may be assigned D configuration with respect to the top COOH.
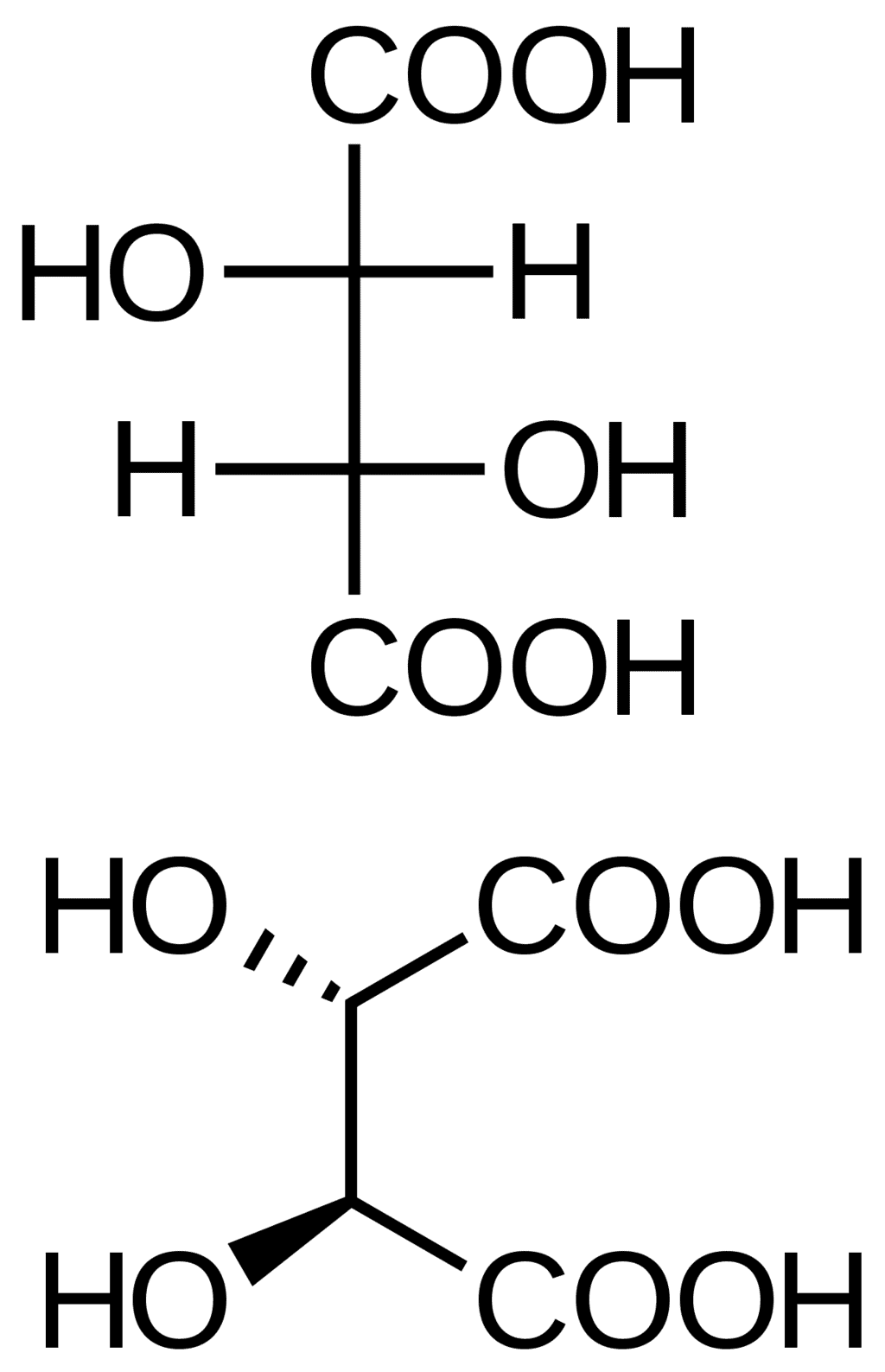 Tartaric Acid
Tartaric Acid
- The D, L system specifies the configurations of only one chiral atom. The configurations at the other chiral centers must be memorized.
- The assignment of configurations to amino acids presented difficulties which resulted in the development of amino acid nomenclature.
R,S System of Nomenclature
According to the Cahn-Ingold-Prelog system, there are a set of rules that can be used for unambiguously defining the stereochemical configuration of any stereocenter. In this R is for Latin rectus which means right-handed and S is for Latin sinister which means left-handed.
An unambiguous and universally applicable system for specifying the absolute configuration of each chiral center in a molecule was devised by Cahn et al.
Rules of R, S Nomenclature
Rule 1: Assign priorities to the four substituents, with 1 being the highest priority and 4 the lowest. Priorities are based on the atomic number. First, examine at the atoms directly attached to the stereocenter of the compound. A substituent with a higher atomic number takes precedence over a substituent with a lower atomic number. Hydrogen is the lowest possible priority substituent, because it has the lowest atomic number. If the chains are similar, proceed down the chain, until a point of difference. 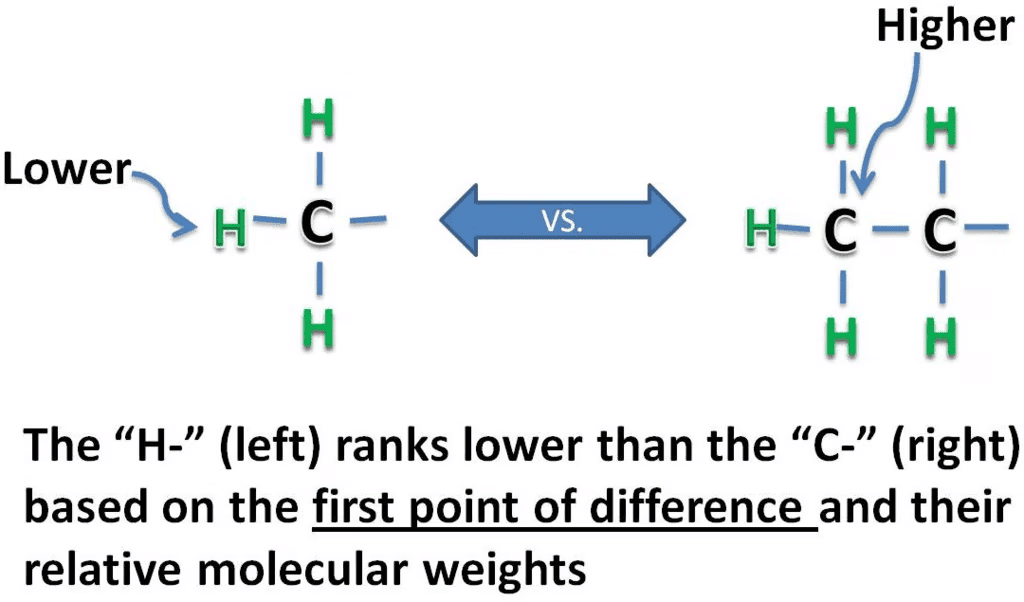
Rule 2: Trace a circle from 1 to 2 to 3.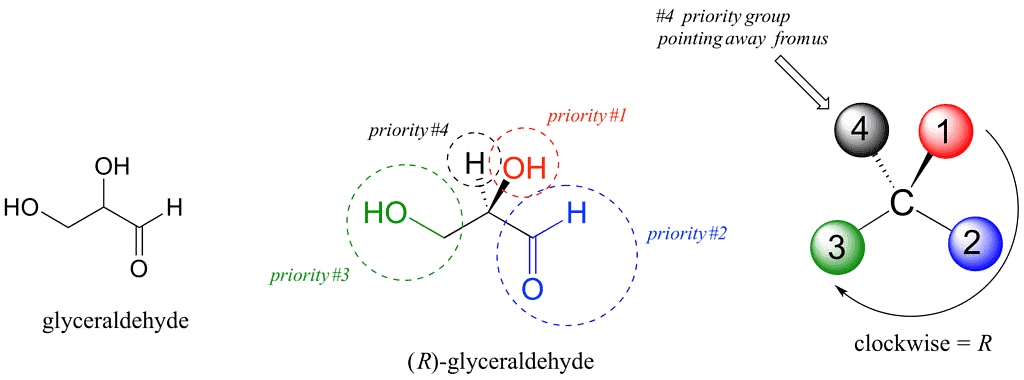
- If two atoms attached to the chiral center are the same the priority is decided by applying the sequence rule to the next atoms in the groups and soon.
- For example: 2-bromobutane two-atom directly attached to the chiral center are carbon. In CH3 the second atoms are H, H, and H in C2H5 they are C, H, H. Since carbon has a higher atomic number so, C2H5 is preferred over CH3.
- If there is a double or triple bond, both double and triple bond atoms are considered to be duplicated or triplicate.
- The priority of cis alkene is higher than trans alkene.
Rule 3: Determine the orientation of the 4 priority group. If it is oriented into the plane of the page (away from you), group pointing away from you a clockwise circle in part 2 corresponds to the R configuration, while a counterclockwise circle corresponds to the S configuration. If it is oriented out of the plane of the page (toward you) group pointing toward you): a clockwise circle in part 2 corresponds to the S configuration, while a counterclockwise circle corresponds to the R configuration. 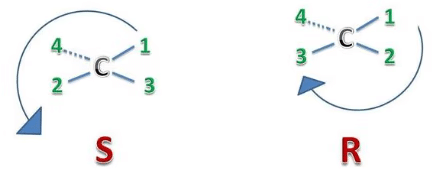
Comparison between D, L, and R, S nomenclature:
- The D, L system of nomenclature is for the nomenclature of relative configuration whereas the R, S system is for the nomenclature of absolute configuration.
- There is no relation between these two systems of nomenclature and sign of rotation. All the D compounds may not have R and all the L compounds may not have S configurations.
Erythro and Threo Nomenclature
Erythro and threo are common terms in stereochemistry used for naming molecules with two stereogenic centers. The names derive from the saccharides erythrose and threose.
- An enantiomer of the one pair is diastereomeric with that of the other pair.
- This nomenclature is based on aldotetrose, erythrose, and threose which exist as two enantiomeric pairs.
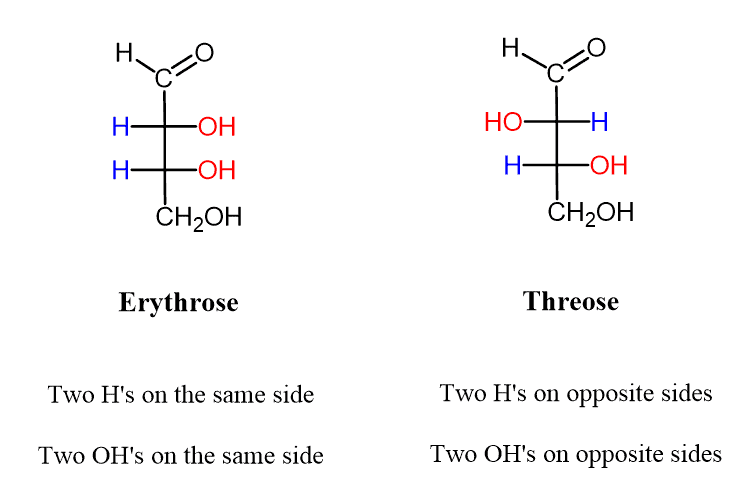
- The Erythro isomer has similar groups at two adjacent chiral centers on the same side of the Fischer projection, while the Threo isomer has the corresponding groups on opposite sides of the Fischer projection.
- This approach for naming chiral compounds became quite general and spread outside of carbohydrates.
- The configuration of any molecule with two stereogenic centers can be classified as erythro or threo.
- For example, the following halide is said to be erythro when the Cl and Br groups on the neighboring carbon atoms are on the same side, and threo, when they are on the opposite sides:
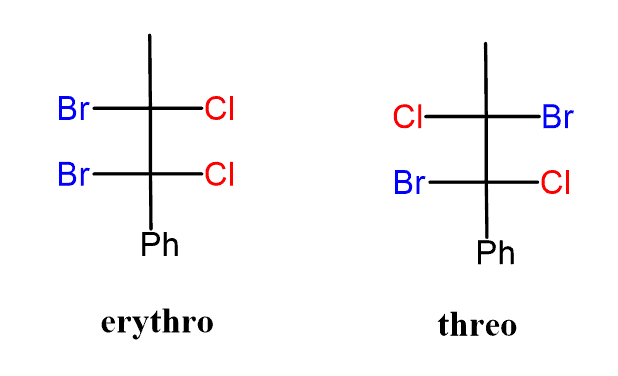
Comparison between Erythro-Threo and Meso-DL pair
For Erythro and Threo nomenclature.
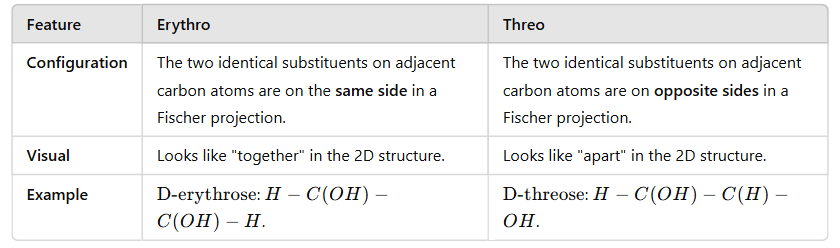
For Meso and DL pair
Cis-Trans Nomenclature for Geometrical Isomers
cis/trans nomenclature is effective only when the alkene has two different groups on each carbon atom of the double bond and each carbon has one of the same group.
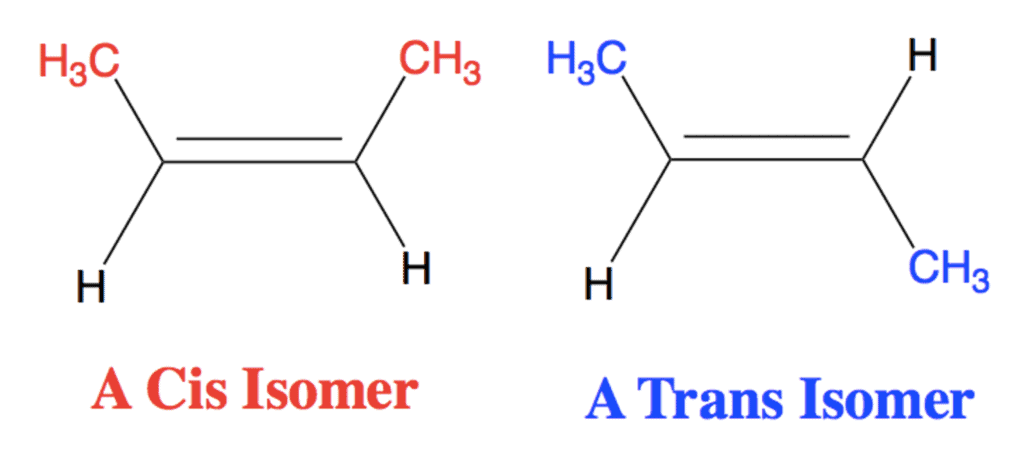
- The isomers in which the identical groups are on the same side of the double bond is called Cis and the isomer in which the identical groups are on the opposite sides is called trans.
- This type of nomenclature can be used only when two or three types of ligands are attached around the double bond.
E-Z Nomenclature for Geometrical Isomers
E-Z notation is used to name geometric isomers. It is an extension of cis-trans isomer. When none or most of the groups attached to the double bond are not same then in IUPAC system geometric isomers are notified or named with E or Z.
- E-Z is based on the cahn-ingold-prelog system. In the E-Z system, the group of highest priority on each carbon atom is identified by using the sequence rules.
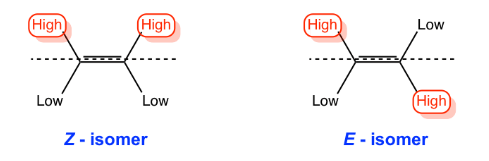
- If the highest priority groups are on the same side of the double bond, the configuration is Z (zusammen=together) and if they are on the opposite sides the configuration is E (entegegen=opposite).
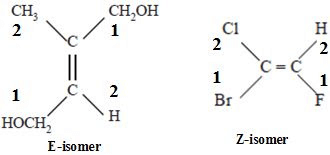
The guidelines for assigning group priority in E/Z naming system
1. Priority is assigned based on the atomic number of the atoms bonded directly to the sp2 double bond carbon – the larger the atomic number, the higher the priority (isotopes with a higher mass number have higher priority). For example: S > O > N > C > H.
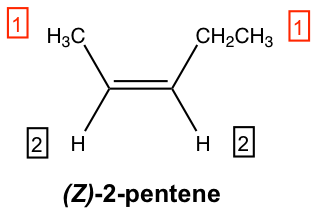
For the above structure of 2-pentene: on the left-side sp2 carbon, the methyl group CH3 is higher than the hydrogen atom because C > H; on the right-side sp2 carbon, the ethyl group CH2CH3 is also higher than hydrogen. With higher priority groups on both sides of the double bond, this is the Z isomer, and the complete name of the compound is (Z)-2-pentene.
The group with higher priority is labelled as 1, and the group with lower priority is labelled as 2 .
2. If the two groups bonded directly on an sp2 carbon start with the same atom, it means there is a tie from step 1. Then, we move on to the atoms connected to the “tied” atom, and priority increases as the atomic number of the next attached atom increases.
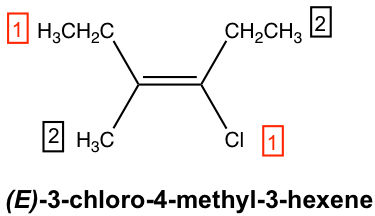 For the above structure, it is obvious that Cl is higher than C (C of CH2CH3 group) on the right-side sp2 carbon.
For the above structure, it is obvious that Cl is higher than C (C of CH2CH3 group) on the right-side sp2 carbon.
On the left-side sp2 carbon, we need to compare between the methyl CH3 group and ethyl CH2CH3 group. Both groups have carbon atoms attached directly to the sp2 carbon, which is a tie. In the CH3 group, the carbon atom is bonded to H, H, H, while in the CH2CH3 group the carbon atom is bonded with H, H, C. So, ethyl CH2CH3 as higher than methyl CH3. Since the higher priority groups are on the opposite side of the double bond, this is the E isomer, and the complete name of the compound is (E)-3-chloro-4-methyl-3-hexene.
Note 1: For this round of comparison between H, H, H and H, H, C, compare the single atom with the greatest number in one group versus the single atom with the greatest number in the other group. So, it is H in one group versus C in the other group, since C > H; therefore, CH2CH3 is higher than CH3. Remember, do not add the atomic numbers. For example, if one group has C, C, C, and the other group has C, O, H, then the C, O, H side is higher because O is higher than C.
Note 2: The above compound is a cis-isomer if using the cis/trans naming system (both ethyl groups are on the same side of the double bond), but it is an E-isomer for the E/Z system. So, cis/trans and E/Z are two different naming systems, but they do not always match.
3. Repeat step 2 if necessary until the priority is assigned.
|
44 videos|102 docs|52 tests
|
FAQs on Stereo: D/L, R/S , Erythro/Threo Nomenclature of Organic Compounds - Organic Chemistry
| 1. What is the D,L system of nomenclature and how is it applied? |  |
| 2. What is the R,S system of nomenclature and how does it differ from D,L? |  |
| 3. What distinguishes erythro and threo nomenclature in organic chemistry? |  |
| 4. How do meso compounds relate to the D,L system? |  |
| 5. What is the difference between cis-trans and E-Z nomenclature for geometrical isomers? |  |

















