Stereochemically Active And Inactive Lone Pairs - Chemical Bonding | Inorganic Chemistry PDF Download
| Table of contents |

|
| Stereochemically Active Lone Pairs |

|
| Stereochemically Inactive Lone Pairs |

|
| Atomic Inversion |

|
| Berry Pseudorotation |

|
Stereochemically Active Lone Pairs
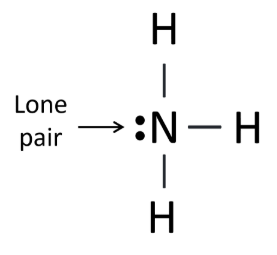
Lone pairs significantly influence the geometry of a molecule, contributing to its shape. These lone pairs occupy space around the central atom, distorting the bond angles and altering the molecular geometry.
Examples:
- Ammonia (NH3): The lone pair on nitrogen causes the molecule to adopt a trigonal pyramidal shape rather than a planar shape.
- Water (H2O): The two lone pairs on oxygen lead to a bent shape, distorting the ideal bond angle of 109.5° to about 104.5°.
- Sulfur Hexafluoride (SF4): The lone pair on sulfur makes the molecule adopt a seesaw shape.
Stereochemically Inactive Lone Pairs
Lone pairs that do not influence the geometry of a molecule significantly and are considered "hidden" in the electronic structure. These lone pairs are not positioned in a way that alters the molecular shape or geometry, often due to their interaction with other orbitals.
Examples:
- Lead(II) compounds (Pb2+): The lone pair on lead is often stereochemically inactive in some compounds like lead(II) chloride (PbCl2).
- Mercury(II) compounds (Hg2+): The lone pair on mercury does not contribute to any distortion of geometry in compounds like mercury(II) chloride (HgCl2).
- Xenon Tetrafluoride (XeF4): The two lone pairs on xenon are positioned opposite each other, canceling their stereochemical effect and leading to a square planar geometry.
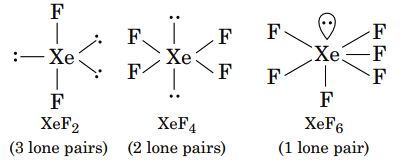
Factors Determining Lone Pair Activity
Orbital Hybridization: Lone pairs in sp3 or sp2 hybridized orbitals are more likely to be stereochemically active. Lone pairs in non-hybridized orbitals (e.g., s-orbitals) may be inactive.
Central Atom: Heavy atoms (like lead or mercury) often have lone pairs that are stereochemically inactive due to relativistic effects or inert pair effects.
Symmetry and Geometry: Symmetrical arrangements can render lone pairs inactive.
Bonding Environment: Lone pairs involved in delocalization or interactions with other atoms (e.g., hydrogen bonding or coordination) may alter their activity.
Understanding stereochemically active and inactive lone pairs helps explain molecular shapes, bond angles, and reactivity in chemistry.
Nature of the lone pair in SbBr63–, TeCl62– and TeBr62–: In these compounds, steric crowding is sufficiently high thus lone pair will be forced to remain in unhybridised s-orbital inside the valence shell. This lone pair is stereochemically inactive.
Nature of lone pair in XeF6: In case of flourides the steric hindrance is relatively less.
Thus the lone pair in XeF6 is in the border line in terms of the properties of the stereochemically active and inactive lone pairs. 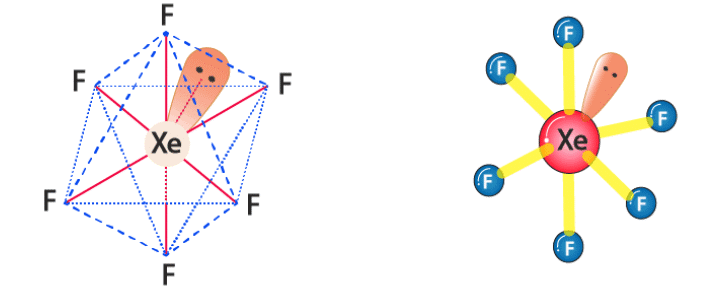
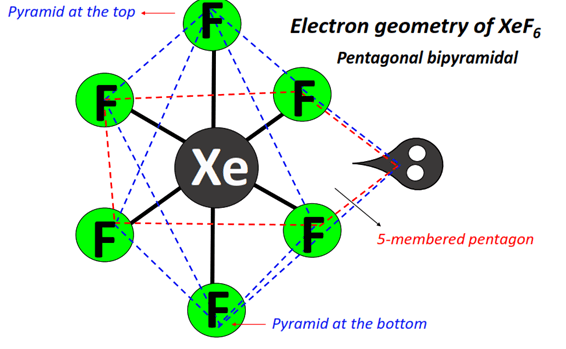
If the lone pair is considered to be stereochemically inactive, then XeF6 should have the perfect octahedral structure and if lone pair is consider to be chemically active then pentagonal bipyramid geometry is considered.
Structure of XeF6 in the solid state: In solid state, no discrete XeF6 molecule exists and the solid consists of square pyramidal XeF5+ cations extensively bridged by free fluoride ions. In such square pyramid moiety, xenon sits at the basal plane.
However, the bridging fluorides maintain a good deal of covalency. Through F-bridging, hexameric, tetrameric and trimeric rings of XeF5+ F– are produced. XeF5+ is expected to be of square pyramid shape. 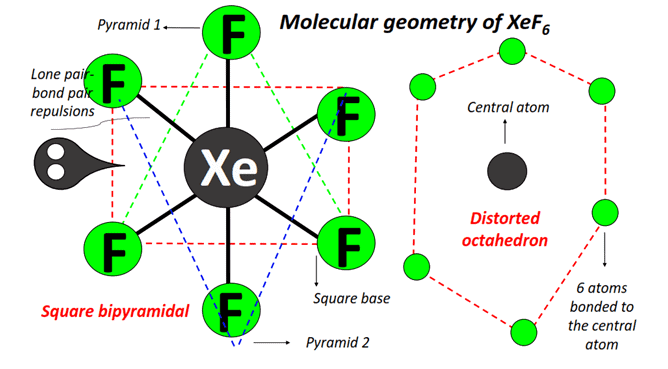 Structure of XeF6 in vapour phase: In the vapour phase, it exists as a monomer, XeF6 which appears to have a slightly distorted octahedral structure but the distortion is less than expected from VSEPR theory. Another important characteristic feature is its fluxional character. If XeF6 is considered to possess a stereochemically active lone pair, then it has got three possibilities, i.e.
Structure of XeF6 in vapour phase: In the vapour phase, it exists as a monomer, XeF6 which appears to have a slightly distorted octahedral structure but the distortion is less than expected from VSEPR theory. Another important characteristic feature is its fluxional character. If XeF6 is considered to possess a stereochemically active lone pair, then it has got three possibilities, i.e.
(i) capped trigonal prism,
(ii) pentagonal bipyramid having the lone pair projected along one corner of the equatorial or basal plane (in terms of octahedreon, the lone pair is projected through the mid point of an edge); and
(iii) capped octahedron (the lone pair is projected through the centre of a trigonal face of an octahedraon, i.e. the lone pair lies along the C3 axis). Thus in no way, it can have a regular geometry. Thus all three structures remains in equilibrium with each other.
XeF6 vs XeF82–: Here, the steric crowding is sufficiently high (compared to XeF6) to convert the lone pair into a stereochemically inactive one. In fact, it adopts the square antiprism where the lone pair contributes nothing to determine the molecular shape.
XeF6 + 2CsF → Cs2[XeF8]
Atomic Inversion
In the simplest reaction a molecule such as ammonia can undergo the inversion of the hydrogen atoms about the nitrogen atom, analogous to the inversion of an umbrella in a high wind.
Consider the trisubstituted amines and phosphines shown in Fig. Because these molecules are nonsuperimposable upon their mirror images (i.e., they are chiral), they are potentially optically active, and separation of the enantiomers is at least theoretically possible.
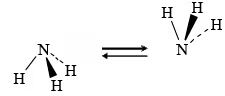
It is of interest that the energy barrier to inversion is strongly dependent on the nature of the central atom and that of the substituents. For example, the barrier to inversion of ethylpropylphenyl-phosphine is about 120 kJ mol–1.
This is sufficient to allow the separation of optical isomers, and their racemization may be followed by classical techniques.
In contrast, the barrier to inversion in most amines is low (~ 40 kJ mol–1 in methyl-propylphenylamine; about 25 kJ mol–1 in ammonia). With such low barriers to inversion, optical isomers cannot be separated because racemization takes place faster than the resolution can be effected.
Highly strained rings such as that shown in Fig. would inhibit inversion. The presence of electron-withdrawing substituents tends to increase the height of the barrier, but electron-donating groups can lower it.
Berry Pseudorotation
Berry pseudorotation is a process in molecules with a trigonal bipyramidal or pyramidal geometry where the positions of ligands (atoms or groups) around a central atom change through a cyclic rearrangement without breaking any bonds.
In this process:
- Ligands move between axial and equatorial positions, but the bonding remains intact.
- It typically occurs in molecules like PF₅ (phosphorus pentafluoride).
- The molecule temporarily passes through a planar transition state, but no bond formation or breaking happens.
This motion is called pseudorotation because it's not a simple rotation; it's an exchange of positions among equivalent sites.
In PF5 the fluorine atoms are indistinguishable by means of 19F NMR. This means that they are exchanging with each other faster than the NMR instrument can distinguish them. The mechanism for this exchange is closely related to the inversion reaction we have seen for amines and phosphines.
The exchange is believed to take place through the conversion of the ground state trigonal bipyramid (TBP) into a square pyramidal (SP) transition state and back to a new TBP structure. 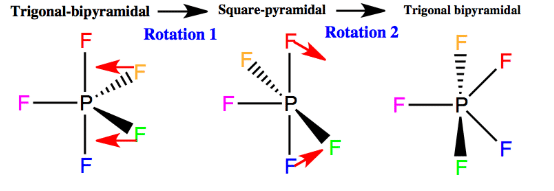
This process results in the complete scrambling of the fluorine atoms at the equatorial and axial positions in phosphorus pentafluoride, and if it occurs faster than the time scale of the NMR experiment (as it does), then all of the fluorine atoms appear to be identical. The entire process is called a Berry pseudorotation.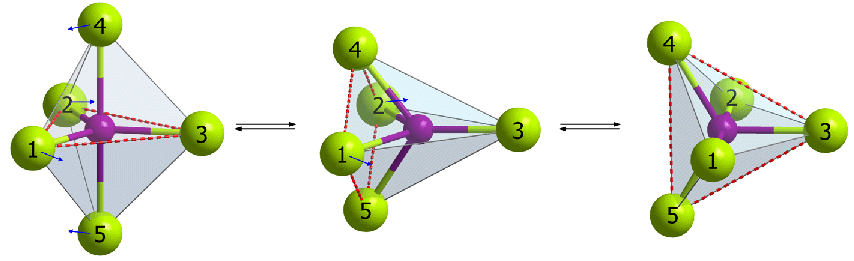 Fig. Berry pseudorotation The exchange of fluorine atoms in PF5 is too rapid to monitor with NMR spectroscopy. The atoms in some other molecules exchange more slowly, especially at lower temperatures.
Fig. Berry pseudorotation The exchange of fluorine atoms in PF5 is too rapid to monitor with NMR spectroscopy. The atoms in some other molecules exchange more slowly, especially at lower temperatures.
For example, PCl2F3 is expected to be a trigonal bipyramid with two apicophilic fluorine atoms in the axial positions, two chlorine atoms, and the third fluorine in equatorial positions.
- At temperatures of –22ºC and above, the resonance of fluorine is observed as a single doublet. However, if the temperature is lowered to –143ºC, the two axial fluorine atoms can be distinguished from the single equatorial fluorine.
- The substitution of alkyl groups on the phosphorus atom provides some interesting effects.
- If a single methyl group replaces a fluorine atom, it occupies one of the equatorial positions as expected and rapid exchange of the two axial and the two equatorial fluorine atoms is observed, as in PF5.
- If two methyl groups are present. (CH3)2PF3, the molecule becomes rigid and there is no observable exchange among the three remaining fluorine atoms.
|
50 videos|92 docs|41 tests
|
FAQs on Stereochemically Active And Inactive Lone Pairs - Chemical Bonding - Inorganic Chemistry
| 1. What is the difference between stereochemically active and inactive lone pairs? |  |
| 2. How do stereochemically active lone pairs affect the molecular geometry? |  |
| 3. Can stereochemically inactive lone pairs form bonds with other atoms? |  |
| 4. How can we determine if a lone pair is stereochemically active or inactive? |  |
| 5. Can stereochemically active lone pairs affect the reactivity of a molecule? |  |





















