Structure of NaCl, CsCl, Diamond & Graphite | Inorganic Chemistry PDF Download
| Table of contents |

|
| Ionic Compounds |

|
| What is Ionic Compound? |

|
| Properties of materials |

|
| Graphene and fullerenes |

|
| Graphene |

|
| Predicting physical states |

|
| Bulk properties |

|
Ionic Compounds
What is Ionic Compound?
Ionic compounds can be defined as: The crystalline solids formed by neatly packed ions of opposite charge. Ionic compounds are usually formed when metals react with non-metals.
In other words, ionic compounds held together by ionic bonds as classed as ionic compounds. Elements can gain or lose electrons in order to attain their nearest noble gas configuration. Formation of ions (either by gaining or losing electrons) for the completion of octet helps them gain stability.
In a reaction between metals and non-metals, metals generally loose electrons to complete their octet while non-metals gain electrons to complete their octet. Metals and non-metals generally react to form ionic compounds.
Structure of NaCl
The structure of an ionic compound depends on the relative sizes of the cations and anions. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Ionic solids are held together by the electrostatic attraction between the positive and negative ions.
For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions. The result is a three-dimensional structure of alternate Na+ and Cl– ions. This is a crystal of sodium chloride. The crystal is uncharged because the number of sodium ions is equal to the number of chloride ions. The forces of attraction between the ions hold them in the structures.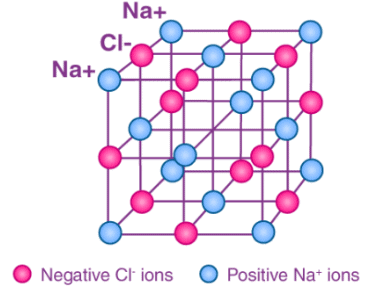 These ionic bonds between the charged particles result in a giant structure of ions. Because the ions are held together tightly in these giant structures it takes a lot of energy to break all the bonds. As a result, ionic compounds have high melting points and boiling points.
These ionic bonds between the charged particles result in a giant structure of ions. Because the ions are held together tightly in these giant structures it takes a lot of energy to break all the bonds. As a result, ionic compounds have high melting points and boiling points.
Ionic Compound Examples
For example reaction between magnesium and chlorine. The magnesium atom has two electrons in its outermost shell. By losing two electrons from its M shell its L shell becomes the outermost shell that has a stable octet. The nucleus of this magnesium atom still has twelve protons but the number of electrons has decreased to ten. So, a net positive charge is developed on this magnesium atom, giving a magnesium cation Mg2+.
On the other hand, the chlorine atom has seven electrons in its outermost shell. Therefore, it needs only one electron to complete its octet. It can gain this one electron from the electrons lost by magnesium atom to become magnesium ion. As two electrons are lost by magnesium atom while one chlorine atom can gain only one electron, two atoms of chlorine combine with one atom of magnesium to form magnesium chloride.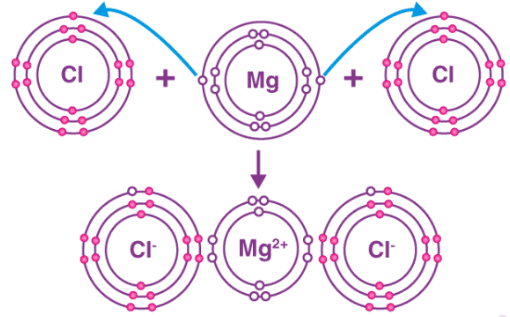 From the above example, ionic compounds can be defined as the compounds formed by the transfer of electrons between metals and non-metals. The bond formed between them is known as the ionic bond. Due to the presence of oppositely charged ions, ionic compounds are held strongly by the electrostatic force of attraction. Prominent properties of ionic compounds are:
From the above example, ionic compounds can be defined as the compounds formed by the transfer of electrons between metals and non-metals. The bond formed between them is known as the ionic bond. Due to the presence of oppositely charged ions, ionic compounds are held strongly by the electrostatic force of attraction. Prominent properties of ionic compounds are:
Ionic Compound Properties
1. Physical properties of ionic compounds
Due to the presence of the strong force of attraction between the positive and negative ions, ionic compounds are solids and are hard to break. They generally break into pieces when pressure is applied, hence they are considered brittle.
2. Melting and boiling points of ionic compounds
Due to the presence of electrostatic forces of attraction between ions, a large amount of energy is required to break the ionic bonds between the atoms. Thus, ionic compounds have high melting and boiling points.
3. The solubility of ionic compounds
Ionic compounds are generally soluble in polar solvents such as water whereas solubility tends to decrease in non-polar solvents such as petrol, gasoline, etc.
4. Conduction of Electricity
Ionic compounds do not conduct electricity in the solid-state but are good conductors in a molten state. Conduction of electricity involves the flow of charge from one point to another. In the solid-state, as the movement of ions is not possible, ionic compounds don’t conduct electricity. Whereas in the molten state, ionic compounds conduct electricity as electrostatic forces of attraction between the ions are overcome by the heat released.
Frequently Asked Questions
➤ Which is an ionic compound?
Ionic compounds are ion compounds. These ions are atoms that gain or lose electrons, resulting in a net positive or negative charge. Metals tend to lose electrons, so they have a net positive charge and become cations. Non-metals tend to gain electrons, creating a net negative charge of anions.
➤ What are the common ionic compounds?
Ionic compounds have high points of melting and boiling and appear to be strong and brittle. Ions may be single atoms, such as sodium and chlorine in common table salt (sodium chloride) or more complex groups such as calcium carbonate.
➤ What is the ionic bond example?
The ionic bond concept is when a positively charged ion forms a bond with a negatively charged ion and one atom passes electrons to another. An example of an ionic bond is Sodium Chloride, a chemical compound.
➤ Is MgO an ionic compound?
To have an octet, Mg loses two electrons. To have an octet, oxygen gains two electrons. The ionic bond between ions is the result of opposite charges being applied electrostatically. The final magnesium oxide formula is MgO.
➤ What are the 2 parts of an ionic compound?
Ionic compounds are compounds made up of ions that form charged particles when an atom (or group of atoms) gains or loses electrons. A cation is an ion charged positively; an anion is an ion charged negatively.
CsCl
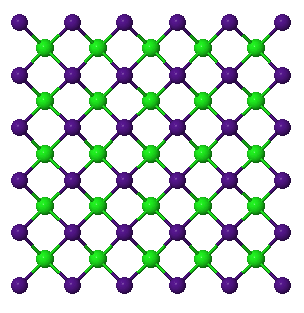 CsCl has a cubic structure that consists of an infinite chain of ions. The Cs (or Cl) ions sit at the eight corners of the cube and the Cl (or Cs) sit at the center of the cube (thus it is NOT a body-centered lattice since that requires the SAME ion to occupy the edges and center). The CN of both cation and anion is 8.
CsCl has a cubic structure that consists of an infinite chain of ions. The Cs (or Cl) ions sit at the eight corners of the cube and the Cl (or Cs) sit at the center of the cube (thus it is NOT a body-centered lattice since that requires the SAME ion to occupy the edges and center). The CN of both cation and anion is 8.
- Hightlight the Cs's
- Highlight the Cl's
- Highlight a Cs site, CN=8
- Highlight a Cl site, CN=8
- wireframe
- Reset
Complexes that share this structure include:
CsBr, CsI, TlCl, TlBr and under high pressure RbCl, RbBr, RbI.
Note that the difference between this and the fluorite structure is that alternate cations are missing in the fluorite lattice.
Properties of materials
Carbon atoms can form four covalent bonds. This lets it form many different organic substances, and to exist as diamond, graphite and fullerenes. Different substances have different bulk properties.
Diamond and graphite
Carbon is an element in group 4 (IUPAC group 14) of the periodic table. Each carbon atom can form four covalent bonds. This means that carbon atoms can form families of similar compounds that have:
- chains
- rings
Organic compounds are substances that contain carbon. There is a vast array of natural and synthetic organic compounds because of the ability of carbon to form four covalent bonds. Carbon can also form giant covalent structures, including diamond and graphite.
Diamond
➤ Structure and bonding
Diamond has a giant covalent structure in which:
- each carbon atom is joined to four other carbon atoms by covalent bonds
- the carbon atoms have a regular lattice arrangement
- there are no free electrons
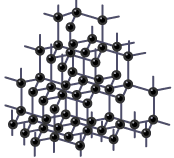 Carbon atoms in diamond have a tetrahedral (pyramid-shaped) arrangement
Carbon atoms in diamond have a tetrahedral (pyramid-shaped) arrangement
➤ Properties and uses
The rigid structure, held together by strong covalent bonds, makes diamond very hard. This physical property makes diamond useful for cutting tools, such as diamond-tipped glass cutters and oil rig drills.
Graphite
➤ Structure and bondingGraphite has a giant covalent structure in which:
- each carbon atom is joined to three other carbon atoms by covalent bonds
- the carbon atoms form layers with a hexagonal arrangement of atoms
- the layers have weak forces between them
- each carbon atom has one non-bonded outer electron, which becomes delocalised
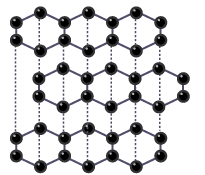 Dotted lines model the weak forces between the layers in graphite
Dotted lines model the weak forces between the layers in graphite
➤ Properties and uses
The delocalised electrons are free to move through the structure, so graphite can conduct electricity. This makes graphite useful for electrodes in batteries and for electrolysis.
The layers in graphite can slide over each other because the forces between them are weak. This makes graphite slippery, so it is useful as a lubricant.
Graphene and fullerenes
Graphene
Graphene is another form of the element carbon. Its structure resembles a single layer of graphite. Graphene has a very high melting point and is very strong because of its large regular arrangement of carbon atoms joined by covalent bonds. Like graphite, graphene conducts electricity well because it has delocalised electrons that are free to move through its structure.
Fullerenes
Fullerenes are forms of carbon, and include nanotubes and buckyballs.
➤ Nanotubes
A nanotube resembles a layer of graphene, rolled into a tube shape. Nanotubes have high tensile strength, so they are strong in tension and resist being stretched. Like graphene, nanotubes are strong, and they conduct electricity because they have delocalised electrons.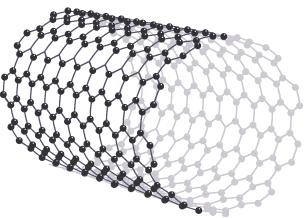 Nanotubes can be several millimetres long but only a few nanometres wide
Nanotubes can be several millimetres long but only a few nanometres wide
➤ Buckyballs
Buckyballs are spheres or squashed spheres of carbon atoms. They are made up of large molecules but do not have a giant covalent structure. Weak intermolecular forces exist between individual buckyballs. Little energy is needed to overcome these forces, so substances consisting of buckyballs are slippery and have lower melting points than graphite or diamond.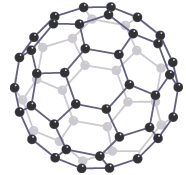 Buckminsterfullerene, C60, has sixty carbon atoms joined by covalent bonds
Buckminsterfullerene, C60, has sixty carbon atoms joined by covalent bonds
Predicting physical states
➤ Melting, evaporating and boiling
Energy must be transferred, by heating, to a substance for these changes of state to happen. During these changes the particles gain energy, which is used to break or overcome:
- some of the bonds between particles during melting
- all of the remaining bonds between particles during evaporating or boiling
Evaporation can take place below the boiling point of a substance. The rate of evaporation is at its maximum at the boiling point.
➤ Condensing and freezing
Energy must be transferred from a substance to the environment for condensation and freezing to happen. During these changes of state the particles lose energy as bonds form between the particles.
➤ Predicting a physical state
The state of a substance at a given temperature can be predicted if its melting point and boiling point are known. The table summarises how to work this out.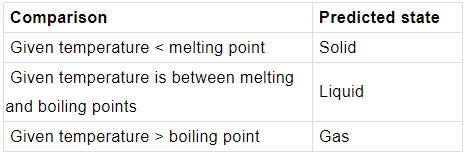
Q : The melting point of oxygen is -218°C and its boiling point is -183°C. Predict the state of oxygen at -200°C.
Ans: Oxygen will be in the liquid state at -200°C (because this is between its melting and boiling points).
Bulk properties
Individual atoms do not have the physical properties of the substances that contain them. For example, a copper atom cannot conduct electricity, even though a piece of copper can do this. Bulk properties are properties due to many atoms, ions or molecules acting together.
➤ Melting and boiling points
Chemical bonds are broken or overcome during melting and boiling. The stronger these bonds are, the higher the melting point and boiling point.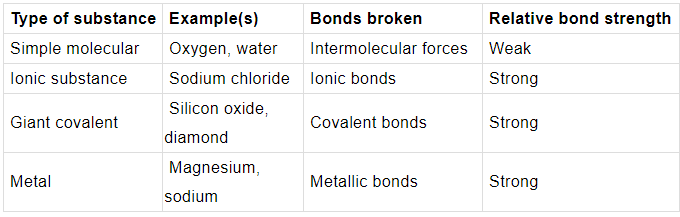
➤ Simple molecular substances:
- have relatively low melting and boiling points
- are usually gases or liquids at room temperature, or solids that are easily melted
➤ Ionic substances, giant covalent substances and metals:
- have relatively high melting and boiling points
- are usually solids at room temperature
Malleable or brittle
Malleable substances can be bent or hammered into shape without shattering. Metals are malleable. When a force is applied, layers of metal ions can slide over each other while still being attracted to the ‘sea’ of delocalised electrons.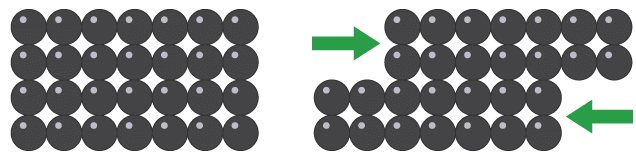 Layers of atoms slide over each other when metals are bent or stretched
Layers of atoms slide over each other when metals are bent or stretched
Ionic substances and giant covalent substances are usually brittle. They shatter when bent or hit because many strong ionic bonds or covalent bonds break at once.
Conducting electricity
A substance can conduct electricity if both:
- it contains charged particles
- these charged particles are free to move about

Note that graphite, graphene and fullerenes are covalent substances that do conduct electricity. This is because, like metals, they contain delocalised electrons.
|
50 videos|92 docs|41 tests
|
FAQs on Structure of NaCl, CsCl, Diamond & Graphite - Inorganic Chemistry
| 1. What is an ionic compound? |  |
| 2. What are the properties of materials? |  |
| 3. What are graphene and fullerenes? |  |
| 4. How can we predict the physical states of substances? |  |
| 5. What is the structure of NaCl, CsCl, diamond, and graphite? |  |















