Separation of Substances Class 6 Notes Science Chapter 3
Separation of Substances
In our daily lives, we often see substances being separated from mixtures. Below are some methods for separating substances.
- A mixture is made up of two or more substances, where each part keeps its own unique properties. For instance, air is a mixture of gases, and the water we drink contains pure water along with other substances.
- A pure substance is one where every particle has the same properties. For example, distilled water is pure because it contains only water.
 We separate substances mainly to extract useful components from mixtures. But why do we need to do this? Sometimes, we need different parts for various uses.
We separate substances mainly to extract useful components from mixtures. But why do we need to do this? Sometimes, we need different parts for various uses.
Examples of Separation Methods
- Tea leaves are removed from the liquid using a strainer when making tea.
- Grain is separated from stalks during harvesting.
- Churning milk or curd helps to separate butter.
- We gin cotton to remove its seeds from the fibre.
- Husk and stones can be separated from grains by handpicking.
- Winnowing separates husk from heavier seeds of grain.
We have studied various methods for separating substances from mixtures, including handpicking, winnowing, sieving, sedimentation, decantation, and filtration.
A solution is formed by dissolving a substance in a liquid. It is termed saturated if it cannot dissolve any more of the substance.
Before using a substance, we need to separate harmful or non-useful components that may be mixed with it. Sometimes, we also need to isolate useful parts if they are needed separately. The substances to be separated may have different sizes or materials, and they can be in any of the three states of matter: solid, liquid, or gas.
So, how do we separate substances that are mixed together if they have many different properties? We will explore some simple methods that you might encounter in everyday activities.
Separating Solid from a Mixture of Solids
Handpicking: This is the easiest way to separate substances. It works best when the unwanted material is small in amount and has a different shape, size, or colour from the useful materials. For instance, handpicking can remove larger impurities like dirt, stones, and husk from wheat, rice, or pulses. It is most effective when the impurities are not too many, such as when removing pebbles, broken grains, and insects from rice, wheat, and pulses.
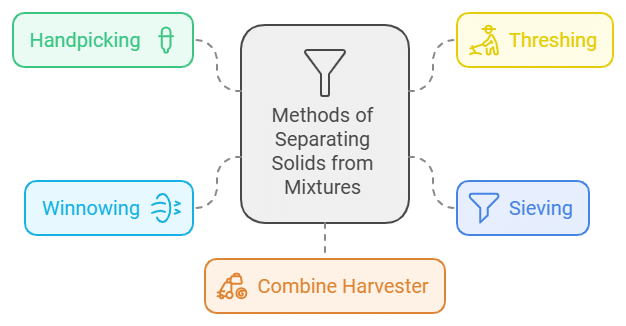
Threshing: This process separates grain from stalks. Before separating the grain, the stalks are dried in the sun. Each stalk has many seeds attached to it, and there can be hundreds of these in a field! Threshing involves beating the stalks to release the seeds.
- Manual Threshing: For small amounts, threshing is done by hand. Small bundles of stalks are thrashed on a hard surface to get the grains out.
- Threshing by Animals: For larger amounts, animals are used. Stalks are placed around a pole, and bullocks walk over them. Their hooves help separate the grains.
- Threshing Machine: Today, machines are often used. These can be powered by diesel engines or electric motors, making the process quicker and easier.
Threshing
Sieving: This method is used when the particles are too small to be picked by hand or when there are too many. A sieve with appropriately sized holes is used. Larger particles stay on the sieve, while smaller ones pass through. For example, it is used to remove impurities from flour or separate sand from gravel.
Winnowing: This process separates lighter particles from heavier ones using wind. It is often used to separate grain from husk. Farmers drop a mixture of wheat and husk from a height. The wind carries the husk away, forming a heap nearby, while the heavier wheat grains fall straight down to form another heap.
 Winnowing
Winnowing
Combine Harvester: Combine harvesters are used in developed countries like the USA to harvest, thresh, and winnow all at once. In India, these machines can be seen on farms in Punjab.
Sedimentation, Decantation & Filtration
 Filtration
Filtration
These methods are commonly used together to separate soluble and insoluble solids from a mixture of solids and liquids.
- Sedimentation: This is when insoluble particles settle at the bottom. For example, in muddy water, soil and sand are impurities that settle down after the water is left to stand.
- Decantation: Used after sedimentation, this method separates the heavier sand particles from water. The clear water is carefully poured off, leaving the sand behind.
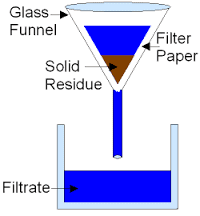
- Filtration: This technique separates fine, insoluble solid particles from liquids. The mixture is passed through a filter, which allows the liquid to flow through while trapping the solid particles.
- Sieving: This method sorts particles based on their sizes. It is often used alongside other separation techniques.
- Evaporation: This process involves converting a liquid into vapour. It is useful for separating a solid that is dissolved in a liquid.
- Condensation: This is the conversion of water vapour back into liquid. Evaporation and condensation work together to separate a soluble solid from water, like separating salt from saltwater.
- Saturated Solution: A saturated solution is one where no more of a substance can dissolve. Heating the solution can allow more substance to dissolve. Different amounts of soluble substances can dissolve in water.
Separation methods often involve multiple techniques to effectively isolate different substances in a mixture. For instance, husk and stones can be removed from grains by handpicking, while heavier seeds can be separated from husk through winnowing.
In summary, methods such as handpicking, winnowing, sieving, sedimentation, decantation, and filtration are effective in separating substances from their mixtures.
|
69 videos|150 docs|104 tests
|
FAQs on Separation of Substances Class 6 Notes Science Chapter 3
| 1. What is the process of sedimentation and how does it work in separating substances? |  |
| 2. How does decantation differ from sedimentation in the separation of mixtures? |  |
| 3. What is filtration and when is it used in the separation of solids? |  |
| 4. Can sedimentation, decantation, and filtration be used together in a separation process? |  |
| 5. What are some real-life applications of sedimentation and filtration? |  |

















