Terpenes -Bio-Molecules | Organic Chemistry PDF Download
Terpenes and terpenoids are the primary constituents of the essential oils of many types of plants and flowers. The odor of a freshly crushed mint leaf, like many plant odors, is due to the presence in the plant of volatile C10 and C15, compounds, which are called terpenes. While terpenoids are compounds derived from a combination of two or more isoprene units. Isoprene is a five carbon unit, chemically known as 2-methyl-1,3-butadiene.
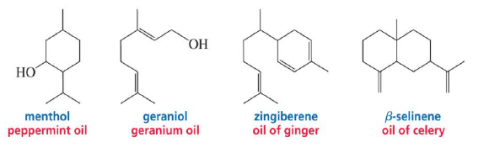
Terpenes - Class of >20,000 compounds containing carbon atoms in multiples of five
Terpenoids - Oxygen-containing terpenes (alcohols, ketones, aldehydes).
Basic structure
The basic molecular formulae of terpenes are multiples of that, (C5H8)n where n is the number of linked isoprene -Isoprene - 2-methyl-1,3-butadiene- units. This is called the isoprene rule or the C5 rule. Compounds containing carbon atoms in multiples of 5 suggest a C5 building block - isoprene units linked in a “head-to-tail” fashion .
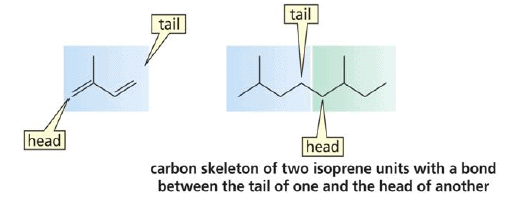
“Head” - branched end of isoprene.
“Tail”- unbranched end of isoprene.
Classification of Terpenes
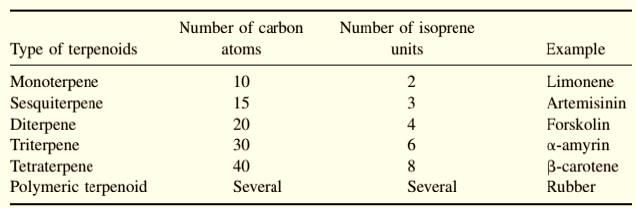
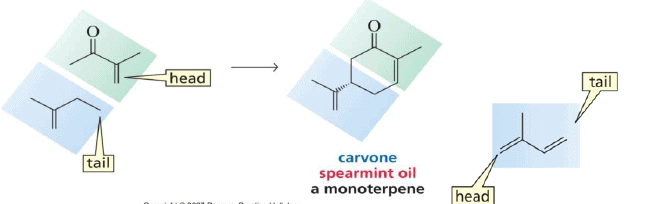
1. Monoterpenes: Consist of two isoprene units and have the molecular formula C10H16. Examples of monoterpenes are: geraniol, terpeniol, limonene.
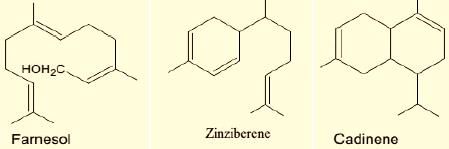
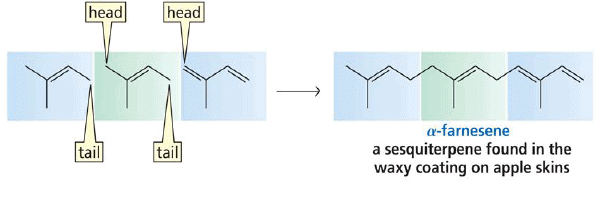
2. Sesquiterpenes: Consist of three isoprene units and have the molecular formula C15H24. Examples of sesquiterpenes: humulene, farnesense, farnesol, zinziberene,cadinene.
 .
.
3. Diterpenes: Composed of four isoprene units and have the molecular formula C20H32. Examples are cafestol, kahweol, cembrene and taxadiene (precursor of taxol). Diterpenes also form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory.
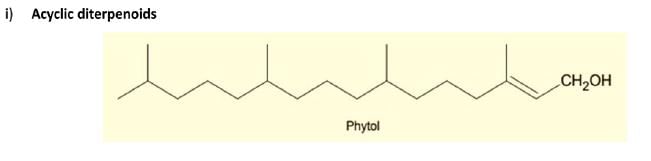
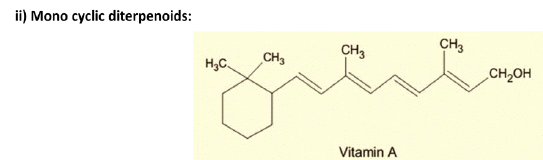
4. Sesterterpenes, terpenes having 25 carbons and five isoprene units, are rare relative to the other sizes. An example of a sesterterpene is geranylfarnesol.
5. Triterpenes consist of six isoprene units and have the molecular formula C30H48. The linear triterpene squalene, the major constituent of shark liver oil.
6. Tetraterpenes contain eight isoprene units and have the molecular formula C40H64. Biologically important tetraterpenes include the acyclic lycopene, the monocyclic gamma-carotene, and the bicyclic alpha- and beta-carotenes.
7. Polyterpenes consist of long chains of many isoprene units. Natural rubber consists of polyisoprene in which the double bonds are cis.
Finding the isoprene building block: cyclic compounds
Finding the isoprene building block: sesquiterpenes (C15)
Finding the isoprene building block: triterpenes (C30) -
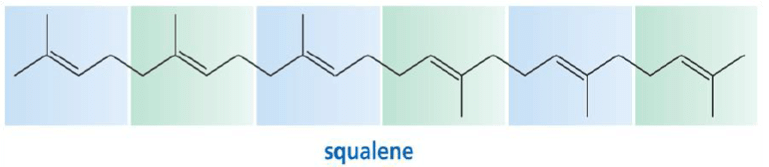
Squalene has a natural and vital part in the synthesis of all plant and animal sterols, including cholesterol, steroid hormones, and vitamin D in the human body.
Finding the isoprene building block: tetraterpenes (C40)
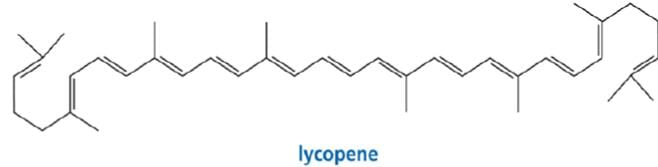
Lycopene's eleven conjugated double bonds give it its deep red color and are responsible for its antioxidant activity. Lycopene is responsible for the red color in tomatoes and watermelon
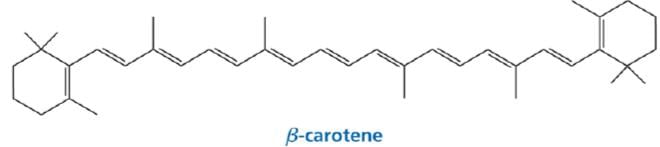
β-carotene is the compound that causes carrots and apricots to be orange.
Isolation of Mono And Sesquiterpenoids
Both mono and sesquiterpenoids have common source i.e essential oils. Their isolation is carried out in two steps:
- Isolation of essential oils from plant parts
- Separation of Terpenoids from essential oils.
(i) Isolation of essential oils from plant parts
The plants having essential oils generally have the highest concentration at some particular time. Therefore better yield of essential oil plant material have to be collected at
this particular time. e.g. From jasmine at sunset. There are four methods of extractions
of oils.
(a) Expression method
(b) Steam distillation method
(c) Extraction by means of volatile solvents
(d) Adsorption in purified fats
Steam distillation is most widely used method. In this method macerated plant material is steam distilled to get essential oils into the distillate form these are extracted by using pure organic volatile solvents. If compound decomposes during steam distillation, it may be extracted with ether at 50oC. After extraction solvent is removed under reduced pressure.
(ii) Separation of Terpenoids from essential oil
A number of terpenoids are present in essential oil obtained from the extraction. Definite hysical and chemical methods can be used for the separation of terpenoids. They are separated by fractional distillation. The terpenoid hydrocarbons distill over first followed by the oxygenated derivatives. More recently different chromatographic techniques have been used both for isolation and separation of terpenoids.
General Properties of Terpenoids
1. Most of the terpenoids are colorless, fragrant liquids which are lighter than water and volatile with steam. A few of them are solids e.g. camphor. All are soluble in organic solvent and usually insoluble in water. Most of them are optically active.
2. They are open chain or cyclic unsaturated compounds having one or more double bonds. Consequently they undergo addition reaction with hydrogen, halogen, acids, etc. A number of addition products have antiseptic properties.
3. They undergo polymerization and dehydrogenation
4. They are easily oxidized nearly by all the oxidizing agents. On thermal decomposition, most of the terpenoids yields isoprene as one of the product.
General Methods of Structure Elucidation of Terpenoids
1. Molecular formula: molecular formula is determined by usual quantitative analysis and mol.wt determination methods and by means of mass spectrometry. If terpenoid is optically active, its specific rotation can be measured.
2. Nature of oxygen atom present: If oxygen is present in terpenoids its functional nature is generally as alcohol aldehyde, ketone or carboxylic groups.
(a) Presence of oxygen atom present: presence of –OH group can be determined by the formation of acetates with acetic anhydride and benzoyate with 3.5-dinitirobenzoyl chloride. Primary alcoholic group undergo esterification more readily than secondary and tertiary alcohols.
(b) Presence of >C=O group: Terpenoids containing carbonyl function form crystalline addition products like oxime, phenyl hydrazone and bisulphite etc. If carbonyl function is in the form of aldehyde it gives carboxylic acid on oxidation without loss of any carbon atom whereas the ketone on oxidation yields a mixture of lesser number of carbon atoms.
3. Unsaturation: The presence of olefinic double bond is confirmed by means of bromine, and number of double bond determination by analysis of the bromide or by quantitative hydrogenation or by titration with monoperpthalic acid.
Presence of double bond also confirmed by means of catalytic hydrogenation or addition of halogen acids. Number of moles of HX absorbed by one molecule is equal to number of double bonds present.
4. Dehydrogenation: On dehydrogenation with sulphur, selenium, polonium or palladium terpenoids converted to aromatic compounds. Examination of these products the skelton structure and position of side chain in the original terpenoids can be determined.
5. Oxidative degradation: Oxidative degradation has been the parallel tool for elucidating the structure of terpenoids. Reagents for degradative oxidation are ozone, acid, neutral or alkaline potassium permanganate, chromic acid, sodium hypobromide, osmium tetroxide, nitric acid, lead tetra acetate and peroxy acids. Since oxidizing agents are selective, depending on a particular group to be oxidized, the oxidizing agent is chosen with the help of structure of degradation products.
6. Number of the rings present: With the help of general formula of corresponding parent saturated hydrocarbon, number of rings present in that molecule can be determined.
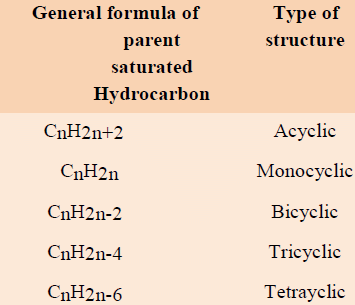
Relation between general formula of compound and type of compounds:
For example: limonene (mol. formula. C10H16) absorbs 2 moles of hydrogen to give tetrahydro limonene (mol. Formula C10H20) corresponding to the general formula. CnH2n. It means limonoene has monocyclic structure.
7. Spectroscopic studies: All the spectroscopic methods are very helpful for the confirmation of structure of natural terpenoids and also structure of degradation products. The various methods for elucidating the structure of terpenoids are;
(a) UV Spectroscopy: In terpenes containing conjugated dienes or α,β-unsaturated ketones, UV spectroscopy is very useful tool. The values of λmax for various types of terpenoids have been calculated by applying Woodward’s empirical rules. There is generally good agreement between calculation and observed values. Isolated double bonds, α,β-unsaturated esters , acids, lactones also have characteristic maxima.
(b) IR Spectroscopy: IR spectroscopy is useful in detecting group such as hydroxyl group (~3400cm–1) or an oxo group (saturated 1750-1700cm–1). Isopropyl group, cis and trans also have characteristic absorption peaks in IR region.
(c) NMR Spectroscopy: This technique is useful to detect and identify double bonds, to determine the nature of end group and also the number of rings present, and also to reveal the orientation of methyl group in the relative position of double bonds.
(d) Mass Spectroscopy: It is now being widely used as a means of elucidating structure of terpenoids for determining mol. wt., mol. formula, and nature of functional groups present and relative positions of double bonds.
8. X-ray analysis: This is very helpful technique for elucidating structure and stereochemistry of terpenoids.
9. Synthesis: Proposed structure is finally confirmed by synthesis. In terpenoid chemistry, many of the synthesis are ambiguous and in such cases analytical evidences are used in conjunction with the synthesis.
|
44 videos|102 docs|52 tests
|
FAQs on Terpenes -Bio-Molecules - Organic Chemistry
| 1. What are terpenes and why are they considered bio-molecules? |  |
| 2. How do terpenes contribute to the flavor and aroma of plants? |  |
| 3. Are terpenes solely found in plants? |  |
| 4. Can terpenes have therapeutic effects on the human body? |  |
| 5. How can terpenes be extracted and used in different industries? |  |

















