Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Theoretical Masses of Products
Theoretical Masses of Products | Chemistry for Grade 10 PDF Download
Obtaining Calculated Masses
Higher Tier Only- We can obtain theoretical masses of products using a balanced equation and a given mass of reactant
- The process is as follows:
- Write out the balanced equation for the reaction(if not already given in the question)
- Convert the given mass of reactant(s) into moles, by dividing the masses by the molar masses
- Use the coefficients(multipliers) in the equation to deduce the number of moles of the product(s)
- Convert the moles of product into mass by multiplying by the molar mass
- The following example illustrates the process:
Solved Example
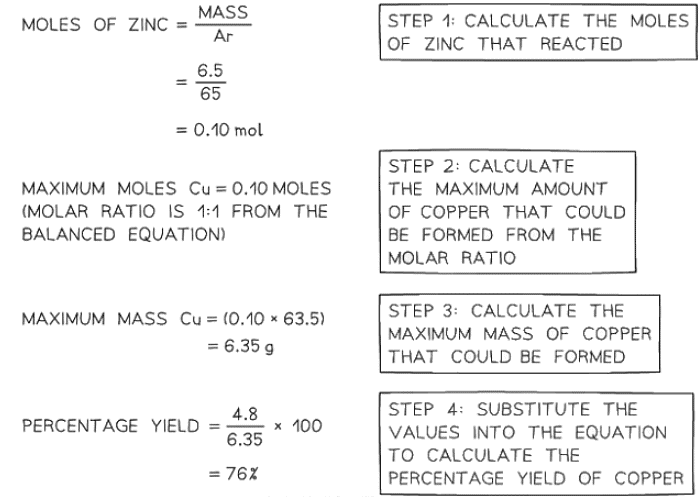
In an experiment to displace copper from copper(II)sulfate, 6.5g of zinc was added to a solution of copper(II)sulfate. The copper was filtered off, washed, dried and weighed. the final mass of copper obtained was 4.8 g. Calculate the percentage yield of copper. The balanced equation for the reaction isZn (s) + CuSO4 (aq) ⟶ Cu (s) + ZnSO4 (aq)
Exam Tip
- The key to success in all calculations is to have a methodical step-by-step approach and show all your workings. Be careful when working out the molar masses not to include coeffiecients. It is very easy to make the mistake of calculating the molar mass of magnesium oxide as 80 g instead of 40 g, when working from an equation:
- 2Mg (s) + O2 (g) → 2MgO (s)
- (Mg = 24 g mol−1; O = 16 g mol−1 )
- Remember that molar masses are calculated from chemical formulae, not from equations
The document Theoretical Masses of Products | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches