UV- Visible Spectroscopy | Organic Chemistry PDF Download
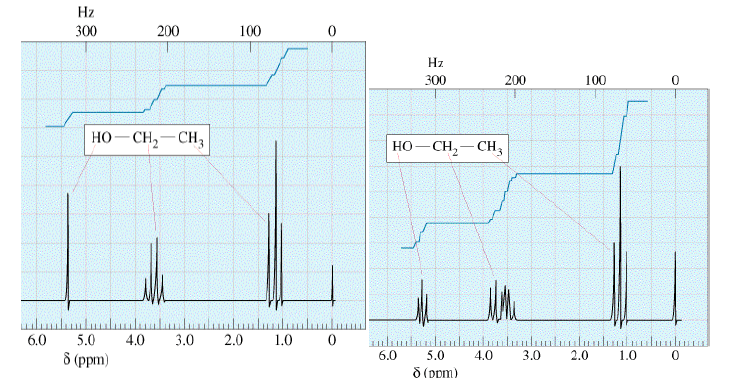
Ultrapure samples of ethanol show splitting (Right). Ethanol with a small amount of acidic or basic impurities will not show splitting (Left).
UV-VISIBLE SPECTROSCOPY
- Provide information about presence and absence of unsaturated functional groups
- Useful adjunct to IR
- Determination of concentration, especially in chromatography
- For structure proof, usually not critical data, but essential for further studies
- NMR, MS not good for purity
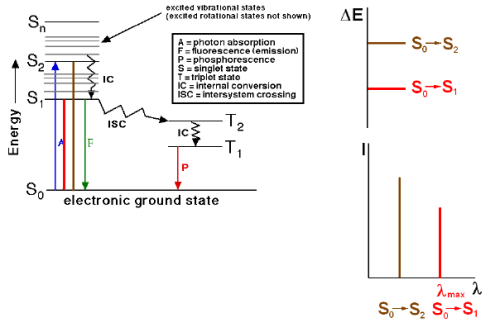
Absorption and Emission
Absorption: A transition from a lower level to a higher level with transfer of energy from the radiation field to an absorber, atom, molecule, or solid.
Emission: A transition from a higher level to a lower level with transfer of energy from the emitter to the radiation field. If no radiation is emitted, the transition from higher to lower energy levels is called Non-radiative decay.
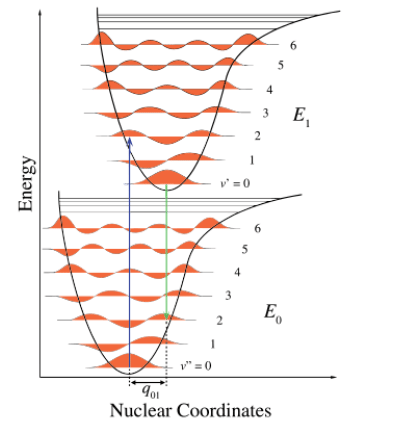
FRANK CODON PRINCIPLE:
(1) The nuclear motion (10–13 s) is negligible during the time required for an electronic excitation (10–16 s).
(2) Since the nuclei do not move during the excitation, the internuclear distances remain constant and “the most probable component of an electronic transition involves only the vertical transitions”.
Absorption and emission pathways
Origin of electronic spectra
Absorption of UV-Visible light by molecule results in electronic excitation of molecule with Chromophore.
Selection Rules
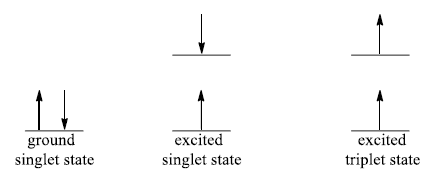
1. Spin selection rule: ΔS = 0
Allowed transitions: singletsinglet or triplettriplet
Forbidden transitions: singlettriplet or tripletsinglet
“Changes in spin multiplicity are forbidden”.
2. Laporte selection rule: There must be a change in the parity (symmetry) of the complex. Electric dipole transition can occur only between states of opposite parity.
Laporte-allowed transitions: g → u or u→ g
Laporte-forbidden transitions: g → g or u → u
g stands for gerade – compound with a center of symmetry
u stands for ungerade – compound without a center of symmetry.
Selection rules can be relaxed due to:
(1).Vibronic coupling (2).Spin-orbit coupling (3).Geometry relaxation during transition
- Spin-forbidden transitions
o Transitions involving a change in the spin state of the molecule are forbidden
o Strongly obeyed
o Relaxed by effects that make spin a poor quantum number (heavy atoms)
- Symmetry-forbidden transitions
o Transitions between states of the same parity are forbidden
o Particularly important for centro-symmetric molecules (ethene)
o Relaxed by coupling of electronic transitions to vibrational transitions (vibronic coupling)
Absorbing species:
(1) π, σ, and n electrons (2) d and f electrons
(3) Charge transfer reactions (4) π, σ, and n (non-bonding) electrons.
Electronic transitions
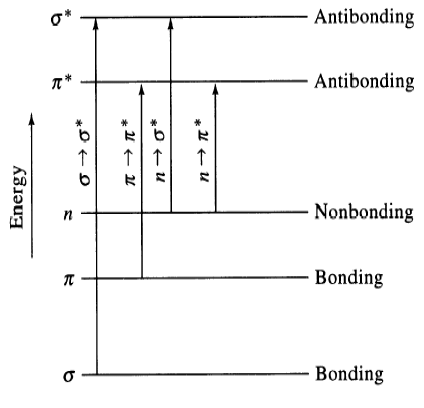
A. σ to σ*
1. UV photon required, high energy
e.g. Methane at 125 nm & Ethane at 135 nm)
B. n to σ*
2. Saturated compounds with unshared e-
- Absorption between 150 nm to 250 nm
- Shifts to shorter wavelengths with polar solvents Minimum accessibility
3. Halogens, N, O, S
C. n to π*, π to π*
4. Organic compounds
5. Requires unsaturated groups (n to π*, π to π*)
D. d-d: 3d and 4d series, broad transitions, impacted by solution
Conventions & Terminologies
Chromophore: A covalently unsaturated group responsible for electronic absorption (e.g. C=C, C=O, esters, amides, —NO2 etc.).
Auxochrome: A saturated group with non-bonded electrons which, when attached to a chromohore, alters both the wavelength and the intensity of the absorption (e.g., —OH, —NH2, —NR2, —SH etc.)
Bathochromic Shift: The shift of absorption band to a longer wavelength. (Also known as “red shift”).
Hypsochromic Shift: The shift of absorption band to a shorter wavelength. (Also known as “blue shift”).
Hyperchromic Effect: An increase in absorption intensity.
Hypochromic Effect: A decrease in absorption intensity.
Woodward Fisher Rule for structure elucidation
Each type of diene or triene system is having a certain fixed value at which absorption takes place; this constitutes the Base value or Parent value. The contribution made by various alkyl substituents or ring residue, double bond extending conjugation and polar groups such as —Cl, —Br etc are added to the basic value to obtain λmax for a particular compound.
I) Conjugated diene correlations:
a) Homoannular Diene:- Cyclic diene having conjugated double bonds in same ring.
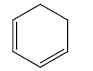
b) Heteroannular Diene:- Cyclic diene having conjugated double bonds in different rings.
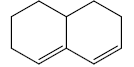
c) Endocyclic double bond:- Double bond present in a ring.
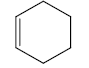
d) Exocyclic double bond: - Double bond in which one of the doubly bonded atoms is a part of a ring system.
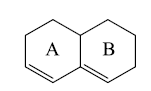
Here, Ring A has one exocyclic and endocyclic double bond. Ring B has only one endocyclic double bond.
Parent values and Increments for different substituents/groups:
(A) Conjugated diene correlations:
1. Base value for homoannular diene = 253 nm
2. Base value for heteroannular diene = 214 nm
3. Alkyl substituent or Ring residue attached to the parent diene = 5 nm
4. Double bond extending conjugation = 30 nm
5. Exocyclic double bonds = 5 nm
6. Polar groups: a) —OAc = 0 nm (b) —OAlkyl = 6 nm (c) —Cl, —Br = 5 nm
Eg: Base value = 214 nm
Ring residue = 3 x 5 = 15 nm
Exocyclic double bond = 1 x 5 = 5 nm
λmax = 214 + 15 + 5 = 234 nm
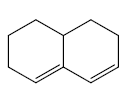
(B) α, β- unsaturated carbonyls:
1. Base value: (a) Acyclic α, β unsaturated ketones = 214 nm
(b) 6 membered cyclic α, β unsaturated ketones = 215 nm
(c) 5 membered cyclic α, β unsaturated ketones = 202 nm
(d) α, β unsaturated aldehydes = 210 nm
(e) α, β unsaturated carboxylic acids & esters = 195 nm
2. Alkyl substituent or Ring residue in α position = 10 nm
3. Alkyl substituent or Ring residue in β position = 12 nm
4. Alkyl substituent or Ring residue in γ and higher positions = 18 nm
5. Double bond extending conjugation = 30 nm
6. Exocyclic double bonds = 5 nm
7. Homodiene compound = 39 nm
8. Polar groups:
(a) –OH in α position = 35 nm
(b)–OH in β position = 30 nm
(a) –OH in δ position = 50 nm
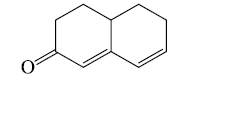
Eg:
Base value = 214 nm
β- Substituents = 1 x 12 = 12 nm
δ- Substituents = 1 x 18 = 18 nm
Double bond extending conjugation =1 x 30 = 30 nm
Exocyclic double bond = 5 nm
λmax = 279 nm
|
44 videos|102 docs|52 tests
|
FAQs on UV- Visible Spectroscopy - Organic Chemistry
| 1. What is UV-Visible spectroscopy and how does it work? |  |
| 2. What are the applications of UV-Visible spectroscopy? |  |
| 3. How is UV-Visible spectroscopy different from infrared spectroscopy? |  |
| 4. What are the advantages of UV-Visible spectroscopy? |  |
| 5. Can UV-Visible spectroscopy be used for quantitative analysis? |  |





















