Understanding Covalent Bonds: Single and Multiple Bonds | General Chemistry for MCAT PDF Download
| Table of contents |

|
| Introduction |

|
| The Octet Rule and Exceptions |

|
| Ionic and Covalent Bonds |

|
| Polar and Non-Polar Covalent Bonds |

|
| Examples of Covalent Bonds |

|
| Covalent Bonds in Molecules |

|
| Conclusion |

|
Introduction
Matter always seeks stability, and for atoms, stability is achieved by following the octet rule. The octet rule states that atoms (with a few exceptions) desire to have 8 electrons in their outermost shell, similar to noble gases. These outermost shell electrons are known as valence electrons. In this article, we will explore the concept of covalent bonds, which play a crucial role in achieving electron stability. We will delve into single and multiple covalent bonds, exceptions to the octet rule, and the characteristics of polar and non-polar covalent bonds.
The Octet Rule and Exceptions
Achieving Stability: The Octet Rule
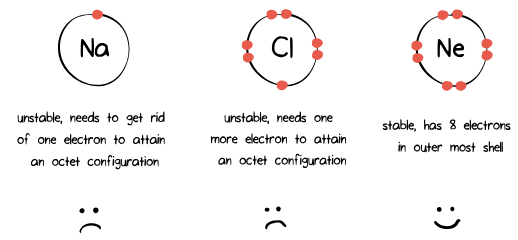
Atoms strive to achieve stability by following the octet rule, which entails having 8 electrons in their outermost shell, except for a few exceptions. Valence electrons play a crucial role in determining an atom's stability.
Exceptions to the Octet Rule
Exceptions to the octet rule include hydrogen (H) and helium (He), which follow the duet rule, accommodating only 2 electrons in their single electron shell. Additionally, some group 3 elements like boron (B) possess three valence electrons. Boron commonly forms three bonds, BH₃, accommodating a total of six electrons in its outermost shell.
Ionic and Covalent Bonds
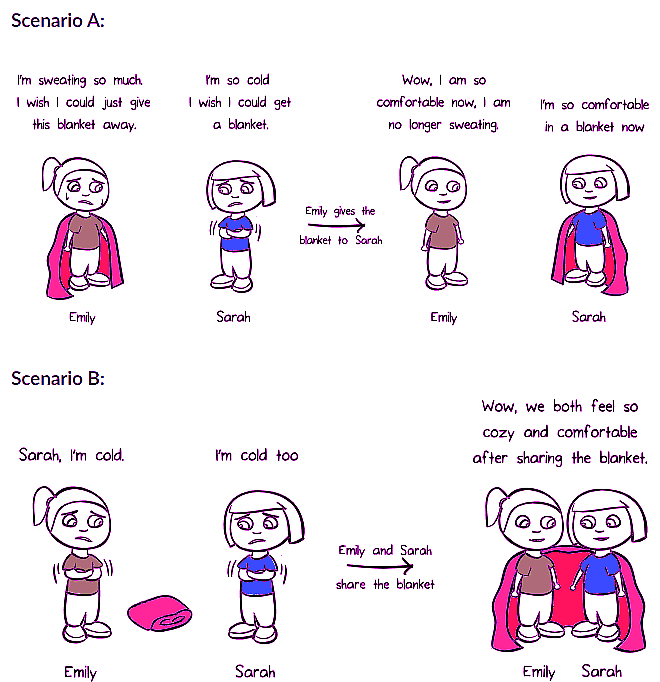
Analogy of Friendship: Ionic and Covalent Bonds
To understand chemical bonding, we can draw an analogy to a friendship between two kids, Emily and Sarah. In scenario A, Emily donates her blanket (electrons) to Sarah, achieving an octet configuration for both. This donation of electrons represents ionic bonding. In scenario B, Emily and Sarah share their blankets, symbolizing a covalent bond.
Electronegativity and Covalent Bonds
Electronegativity, a measure of an atom's ability to attract electrons in a chemical bond, determines the type of covalent bond formed. Electronegativity values vary across the periodic table, with francium being the least electronegative and fluorine being the most electronegative element.
Polar and Non-Polar Covalent Bonds
Non-Polar Covalent Bonds
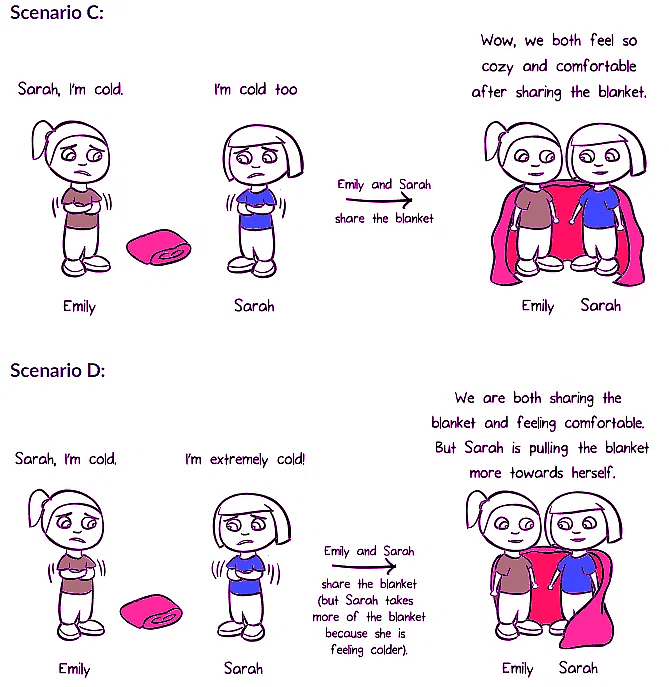
In scenario C, Emily and Sarah are equally cold, indicating that they have the same electronegativity. When atoms with equal electronegativity share valence electrons, a non-polar covalent bond is formed. The electrons are shared equally between the atoms.
Polar Covalent Bonds
In scenario D, Emily is cold, but Sarah is much colder. Sarah pulls the shared blanket (electrons) towards herself, creating polarity in the bond. This represents a polar covalent bond. The more electronegative atom attracts the electrons more strongly, resulting in partial positive and negative charges.
Examples of Covalent Bonds
Non-Polar Covalent Bond Example
Methane (CH₄) serves as an example of a compound with non-polar covalent bonds. Carbon forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons. The electronegativity difference between carbon and hydrogen is below the threshold for polarity.
Single and Multiple Covalent Bonds
The number of electron pairs shared between two atoms determines the type of covalent bond formed. Single, double, and triple covalent bonds arise from the sharing of one, two, and three pairs of electrons, respectively.
Covalent Bonds in Molecules
Triple Covalent Bond
Nitrogen atoms can achieve an octet configuration by sharing three electrons, forming a triple covalent bond. This bond involves the sharing of three pairs of electrons.
Carbon Dioxide and Covalent Bonds
Examining carbon dioxide (CO₂), we can determine the type of covalent bonds it forms based on the arrangement of its atoms.
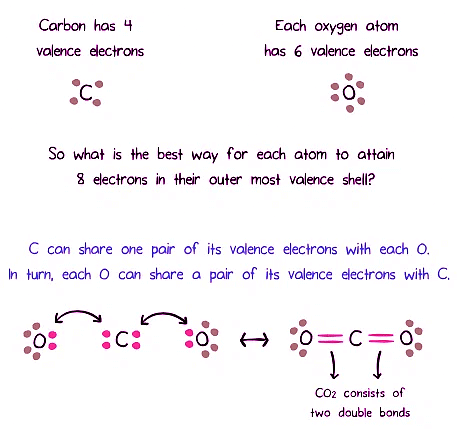
Conclusion
Understanding covalent bonds is essential for comprehending the stability and properties of chemical compounds. The octet rule guides atoms towards stability, with exceptions such as hydrogen and boron. Covalent bonds can be either polar or non-polar, depending on the electronegativity difference between atoms. By sharing valence electrons, atoms form single, double, or triple covalent bonds. Exploring these concepts enables us to grasp the intricacies of molecular structures and their behavior.
|
164 videos|11 docs|16 tests
|



















