VSEPR Theory: Definition, Postulates & Limitations | Chemistry Class 11 - NEET PDF Download
What is VSEPR Theory?
The VSEPR (Valence Shell Electron Pair Repulsion) theory is a model in chemistry that is used to predict the three-dimensional geometry of molecules.
It is based on the idea that electron pairs, whether they are in the form of bonding pairs or lone pairs, repel each other, and the molecular geometry is determined by the arrangement of these electron pairs to minimize their repulsions.
Why VSEPR was introduced?
- The Lewis concept falls short in elucidating molecular shapes, lacking a comprehensive method for predicting covalent molecule geometries.
- In 1940, Sidgwick and Powell introduced a theory rooted in the repulsive interactions among electron pairs within the valence shell of atoms.
- This theory, subsequently refined by Nyholm and Gillespie in 1957, offers a straightforward approach to anticipating the shapes of covalent molecules.
What are the Main Postulates of VSEPR Theory?
The VSEPR (Valence Shell Electron Pair Repulsion) theory is based on a set of postulates that provide the foundation for predicting the three-dimensional geometry of molecules. The main postulates of VSEPR theory are as follows:
- Dependence on Valence Shell Electron Pairs: The shape of a molecule relies on the number of valence shell electron pairs, whether they are bonded or non-bonded, surrounding the central atom.
The following terms are commonly used in discussing the shapes of molecules.
1. Bond Angle: This is the angle between a bonded atom, the central atom, and another bonded atom. 2. Lone Pair: This refers to a pair of valence electrons that are not shared with another atom.
2. Lone Pair: This refers to a pair of valence electrons that are not shared with another atom. Lone Pair and Bond Pair3. Molecular Geometry: This is the 3-D arrangement of bonded atoms in a polyatomic ion or molecule. Molecular geometry does not include unpaired electrons, whereas electron pair geometry includes both bonded atoms and unpaired electrons
Lone Pair and Bond Pair3. Molecular Geometry: This is the 3-D arrangement of bonded atoms in a polyatomic ion or molecule. Molecular geometry does not include unpaired electrons, whereas electron pair geometry includes both bonded atoms and unpaired electrons 4. Electron Pair Geometry: This is the 3-D arrangement of electron pairs around the central atom of a polyatomic ion or molecule.
4. Electron Pair Geometry: This is the 3-D arrangement of electron pairs around the central atom of a polyatomic ion or molecule. - Electron Repulsion in Valence Shell: Electrons within valence shell pairs repel each other due to the negative charge in their electron clouds.
- Optimal Spatial Arrangement for Minimizing Repulsion: Electron pairs naturally organize themselves in space to reduce repulsion and maximize the distance between them.
- Concept of Valence Shell as a Sphere: We visualize the valence shell as a spherical region, with electron pairs distributed on its surface to maximize their separation.
- Treatment of Multiple Bonds: Multiple bonds are treated as single electron pairs, and the electron pairs within multiple bonds are regarded as a combined pair.
- Application of VSEPR Model to Resonance Structures: When multiple resonance structures can represent a molecule, the VSEPR model applies to any of these representations.
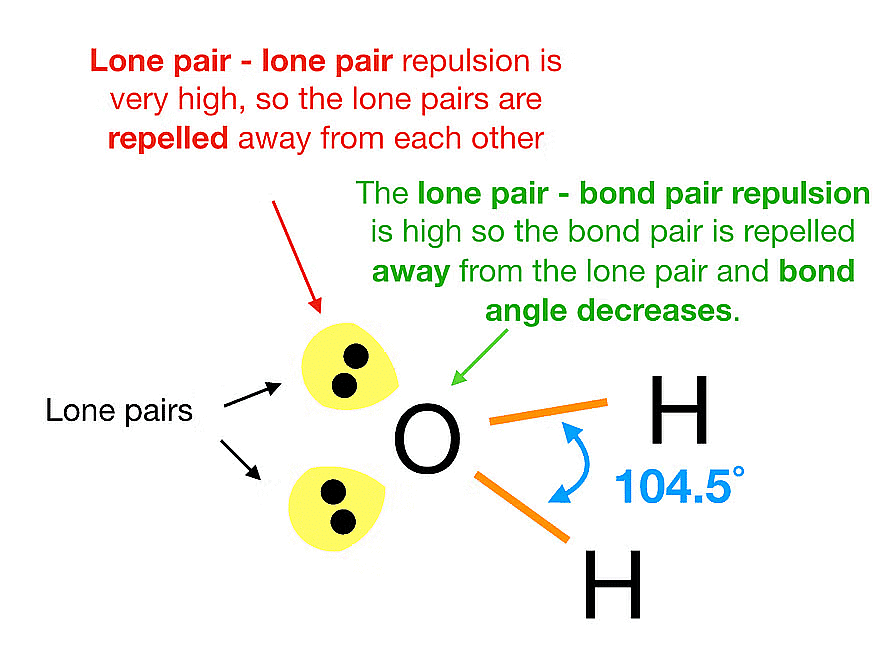
- Hierarchy of Electron Pair Repulsion: The strength of repulsive interactions between electron pairs follows a specific order: Lone pair (lp) – Lone pair (lp) > Lone pair (lp) – Bond pair (bp) > Bond pair (bp) – Bond pair (bp).

Principles of VSEPR Theory
- Electron Pair Arrangement: Electron pairs around a central atom arrange themselves in a way that minimizes their repulsions.
- Variations in Repulsion: Different types of electron pairs, such as bonding pairs and lone pairs, have different levels of repulsion. Lone pairs generally repel more strongly than bonding pairs.
- Geometric Consequences: The repulsion between electron pairs leads to specific geometric arrangements of atoms around the central atom, which result in different molecular shapes.
- According to the VSEPR theory, the repulsion between two electrons is caused by the Pauli exclusion principle which has greater importance than electrostatic repulsion in the determination of molecular geometry.
How to Predict the Shapes of Molecules Using VSEPR Theory?
Steps to apply the VSEPR (Valence Shell Electron Pair Repulsion) procedure:
1. Start by sketching the Lewis electron structure for the molecule or ion.
2. Choose the central atom, opting for the least electronegative one to facilitate effective electron sharing.
3. Count the total bond pairs associated with the central atom, considering the atoms connected by single bonds. Also, tally the electrons in the outermost shell of the central atom.
4. Identify other atoms and their bonds, calculating the total electrons associated with them, including those involved in bonds with the central atom.
5. Determine the optimal arrangement of electron groups around the central atom to minimize repulsions.
6. Recognize interactions between lone pairs (LP–LP, LP–BP, or BP–BP).
7. Calculate the number of lone pairs by subtracting shared pairs from the total electrons.
8. Adjust the valence shell electron pair number (VSEP) for ions by adding or subtracting electrons based on their charges.
9. Anticipate deviations from ideal bond angles.
10. Conclude by describing the final molecular geometry.
What is VSEP Number?
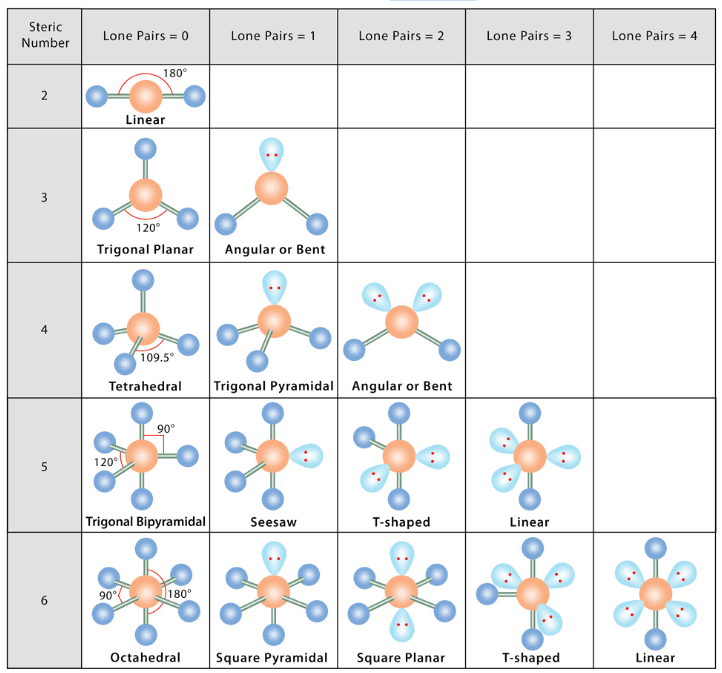
The VSEP number describes the shape of the molecule, as described in the table provided below:
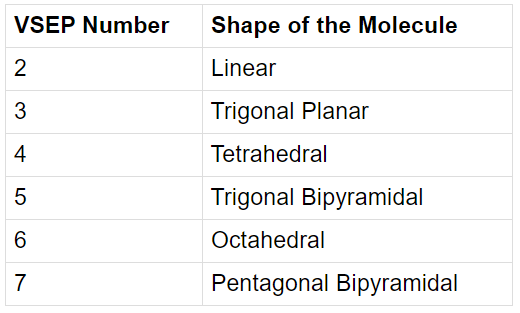 VSEP Number and Shapes
VSEP Number and Shapes
Note: The VSEPR theory cannot be used to obtain the exact bond angles between the atoms in a molecule.
 |
Download the notes
VSEPR Theory: Definition, Postulates & Limitations
|
Download as PDF |
Theoretical Shapes
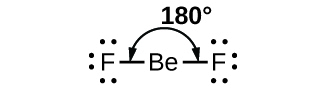
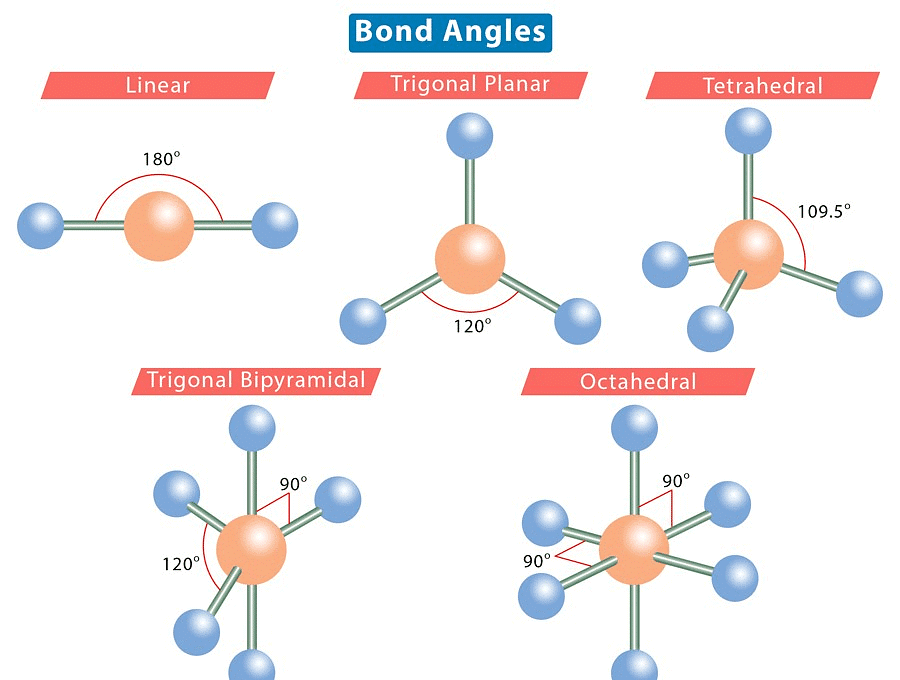
1. Linear Shape of Molecule
- In this type of molecule, we find two places in the valence shell of the central atom.
- They should be arranged in such a manner that repulsion can be minimized (pointing in the opposite direction).
- Example: BeF2
 Molecular structure of BeF2
Molecular structure of BeF2
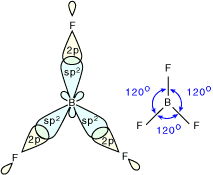
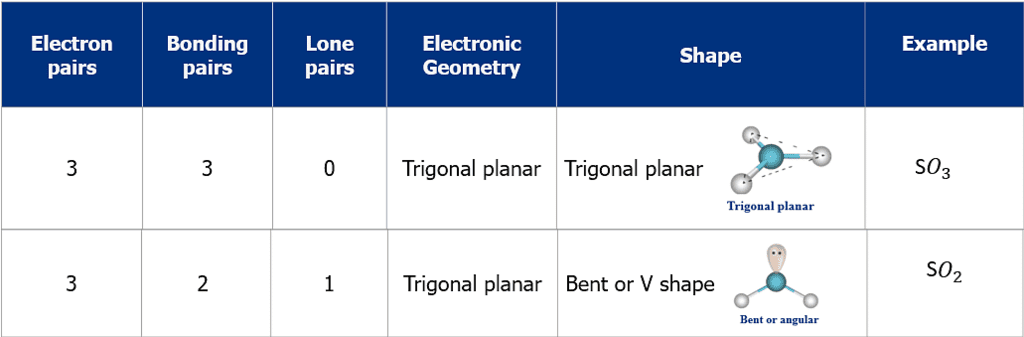
2. Trigonal Planar Shape of Molecule
- In this type of molecule, we find three molecules attached to a central atom.
- They are arranged in such a manner that repulsion between the electrons can be minimized (toward the corners of an equilateral triangle).
- Example: BF3
 Molecular structure of BF3
Molecular structure of BF3
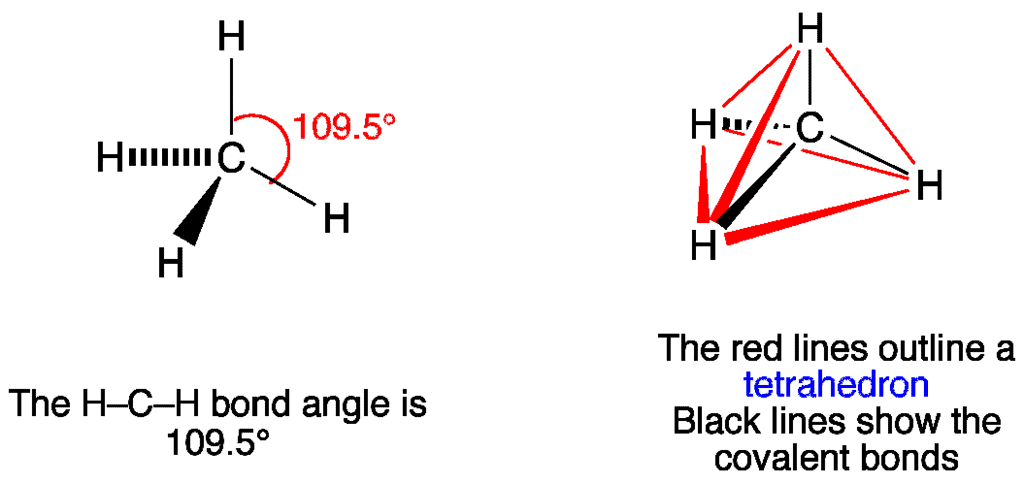
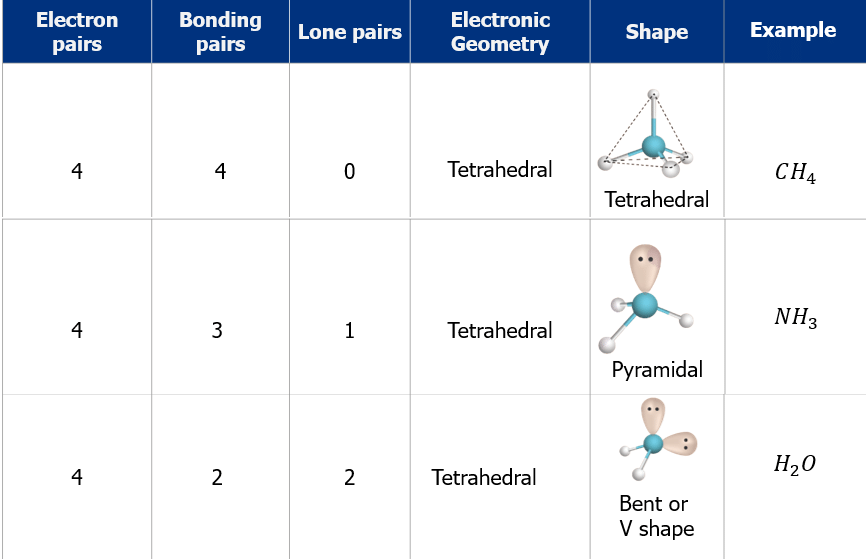
3. Tetrahedral Shape of Molecule
- In two-dimensional molecules, atoms lie in the same plane and if we place these conditions on methane, we will get a square planar geometry in which the bond angle between H-C-H is 90º.
- Now, if we consider all these conditions for a three-dimensional molecule, we will get a tetrahedral molecule in which the bond angle between H-C-H is 109º28’ (toward the corners of an equilateral triangle) CH4.
 Molecular Structure of CH4
Molecular Structure of CH4
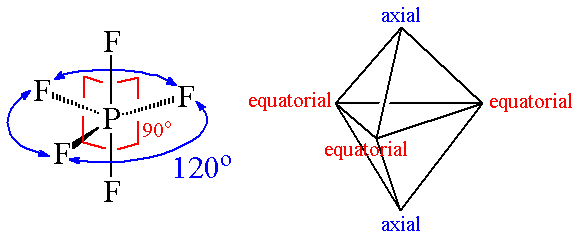
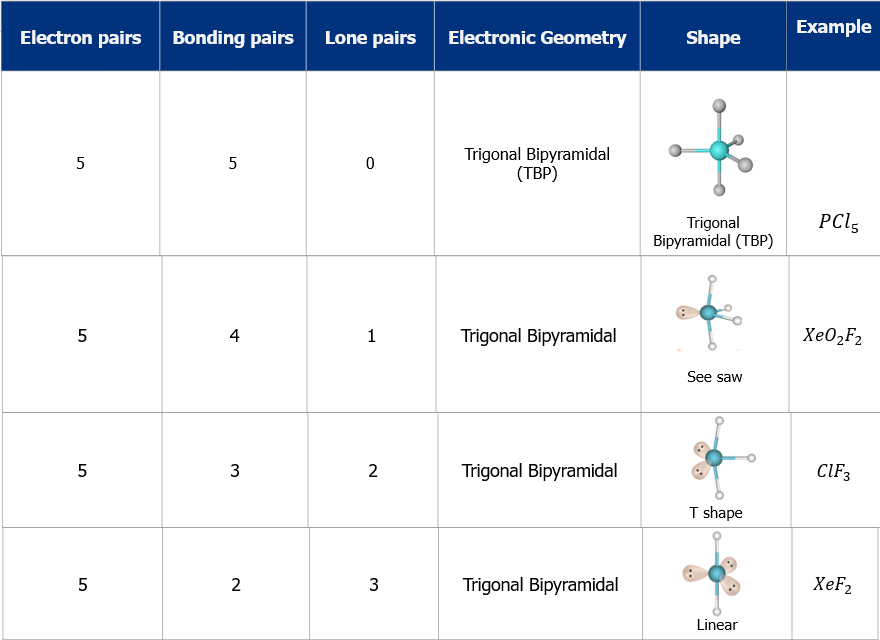
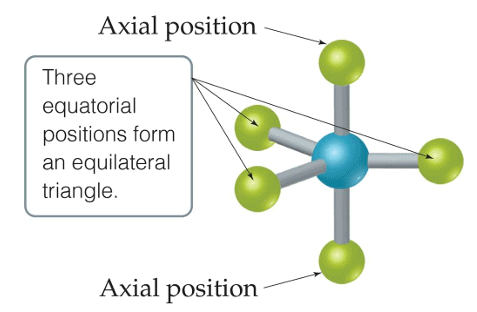
4. Trigonal Bipyramid Shape of Molecule
- For PF5, repulsion can be minimized by an even distribution of electrons toward the corner of a trigonal pyramid.
- In trigonal bipyramid, three positions lie along the equator of the molecule. The two positions lie along an axis perpendicular to the equatorial plane.
 Molecular structure of PF5
Molecular structure of PF5
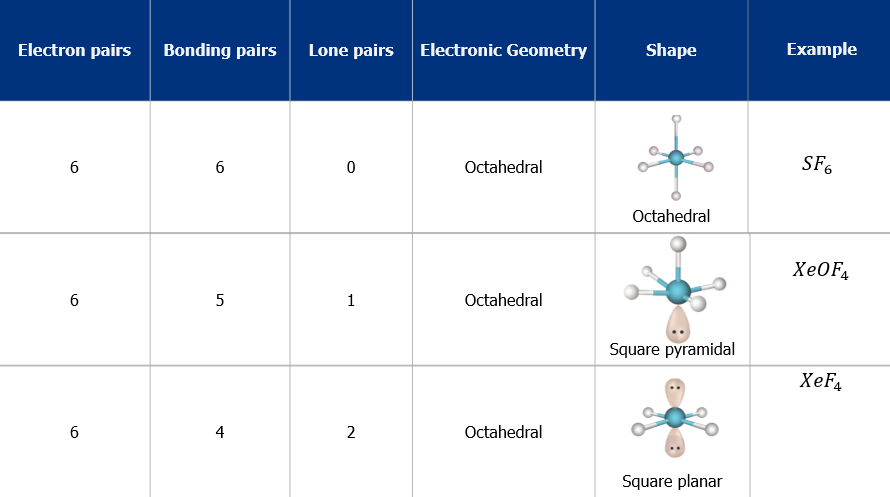
5. Octahedral Shape of Molecule
In Octahedral shape, the central atom attaches 6 different atoms i.e. in total there are 6 bond pairs. These 6 bond pairs and the central atom arrange Octahedral to minimize electron repulsion.
Example of an Octahedral Shape is SF6
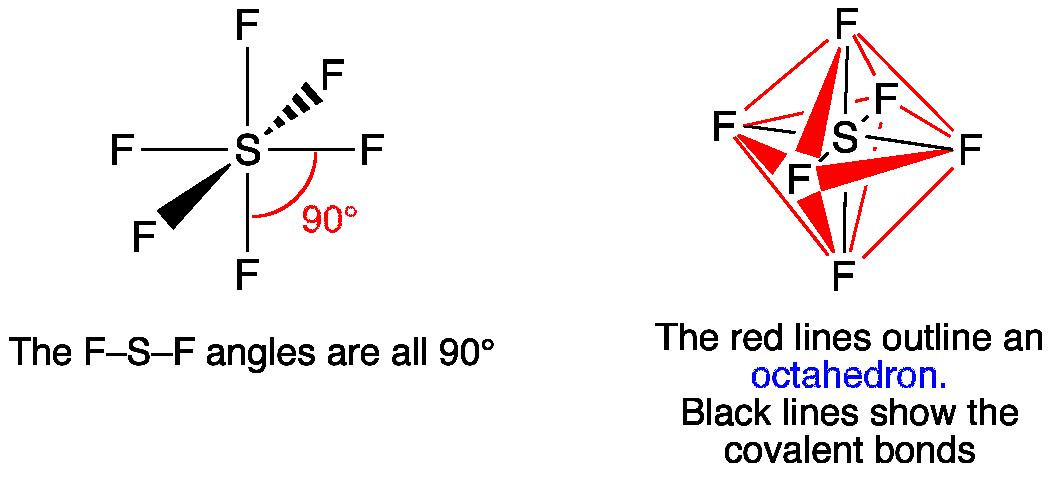 Octahedral Shape of SF65. Pentagonal Bipyramidal Shape of Molecule
Octahedral Shape of SF65. Pentagonal Bipyramidal Shape of Molecule
In a Pentagonal Bipyramidal Shape, the central atom is attached to seven atoms at the corner to minimize the repulsion between the electron pairs.
Example of a Pentagonal Bipyramidal Shape is IF7
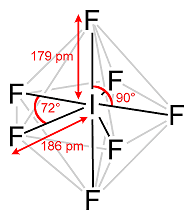 Pentagonal Bipyramidal Shape of IF7
Pentagonal Bipyramidal Shape of IF7
Shapes of molecules based on VSEPR theory
The electron pairs around the central atom repel each other and move so far apart from each other that there are no greater repulsions between them. This results in the molecule having minimum energy and maximum stability.
- The shape of a molecule with only two atoms is always linear.
- For molecules with three or more atoms, one of the atoms is called the central atom, and other atoms are attached to the central atom.
- If the central atom is linked to similar atoms and is surrounded by bond pairs of electrons only, the repulsions between them are similar as a result the shape of the molecule is symmetrical and the molecule is said to have regular geometry.
- If the central atom is linked to different atoms or is surrounded by a bond pair as well as a lone pair of electrons, the repulsion between them is similar. As a result, the shape of the molecule has an irregular or distorted geometry.
- The exact shape of the molecule depends upon the total number of electron pairs present around the central atom.
- The shape and geometry of a molecule with two electron pairs

- The shape and geometry of a molecule with three electron pairs

- The shape and geometry of the molecules with four electron pairs

- The shape and geometry of the molecules with five electron pairs

- The shape and geometry of the molecules with six electron pairs

Limitations of VSEPR Theory
Some significant limitations of the VSEPR theory include:
- This theory fails to explain isoelectronic species (i.e. elements having the same number of electrons). The species may vary in shape despite having the same number of electrons.
- The VSEPR theory does not shed any light on the compounds of transition metals. The structure of several such compounds cannot be correctly described by this theory. This is because the VSEPR theory does not take into account the associated sizes of the substituent groups and the lone pairs that are inactive.
- Another limitation of VSEPR theory is that it predicts that halides of group 2 elements will have a linear structure, whereas their actual structure is a bent one.
Practice Problems on Predicting Shapes via VSEPR Theory
Q.1. How to explain the shape of molecules using VSEPR theory?
Ans:
- The principle of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other, and will, therefore, adopt an arrangement that minimizes this repulsion, thus determining the molecule's geometry.
- The repulsion between two lone pairs is maximum, the repulsion between lone pair and bond pair is intermediate and bond pair-bond pair repulsion is the least.
- According to this, the structures of molecules of different types are:
Q.2. Determine the geometry of the following molecules using the VSEPR model.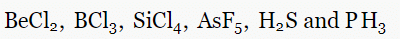
Solution: 
Q.3. The shape of the methane molecule is
A. Tetrahedral
B. Pyramidal
C. Octahedral
D. Square planar
Answer: Methane molecule has 4 electron pairs with zero lone pairs so the shape along with geometry is tetrahedral.
Q 4: Which of the following has trigonal planar geometry?
As we know that the electronic geometry of a molecule depends on the number of bond pairs and lone pairs, while the shape depends only on the number of bond pairs. So, for type AB3 molecule, the shape of the molecule is the same as its geometry, if there is no lone pairs. The repulsions are minimal when electron pairs (bond pairs) are 120° apart from each other (the three bond pairs are farthest from each other when the bond angle B‒A‒B is equal to 120°). The geometry and shape of the molecule are trigonal planar. BF3 molecule has trigonal planar geometry. The central atom B has 3 bond pairs and zero lone pairs of electrons. It undergoes sp2 hybridization. IF3 has trigonal pyramidal geometry, and PCl3 and NH3 have tetrahedral geometry.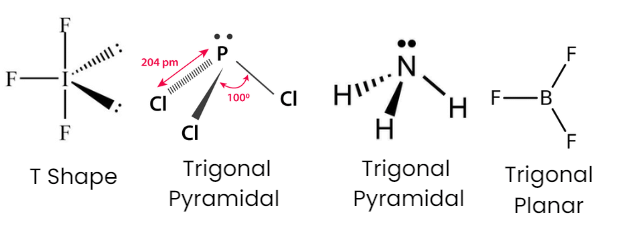
Q.5. Trigonal bipyramidal is not considered a symmetrical geometry. Why?
Answer: Trigonal bipyramidal is not a perfect symmetrical geometry. In this geometry, all the positions are not equivalent. There are two types of positions axial and equatorial. The three bonds in the plane form a trigonal planar-like geometry. These are known as equatorial bonds. There are bonds above and below the plane. These bonds are known as axial bonds. The axial bonds are longer and weaker, while the equatorial bonds are shorter and stronger.
|
127 videos|245 docs|87 tests
|
FAQs on VSEPR Theory: Definition, Postulates & Limitations - Chemistry Class 11 - NEET
| 1. What is VSEPR Theory and why is it important in chemistry? |  |
| 2. What are the main postulates of VSEPR Theory? |  |
| 3. How can you predict the shape of a molecule using VSEPR Theory? |  |
| 4. What are some limitations of VSEPR Theory? |  |
| 5. Can you provide an example of a practice problem involving VSEPR Theory? |  |


 2. Lone Pair: This refers to a pair of valence electrons that are not shared with another atom.
2. Lone Pair: This refers to a pair of valence electrons that are not shared with another atom.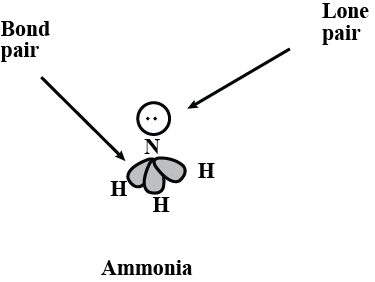
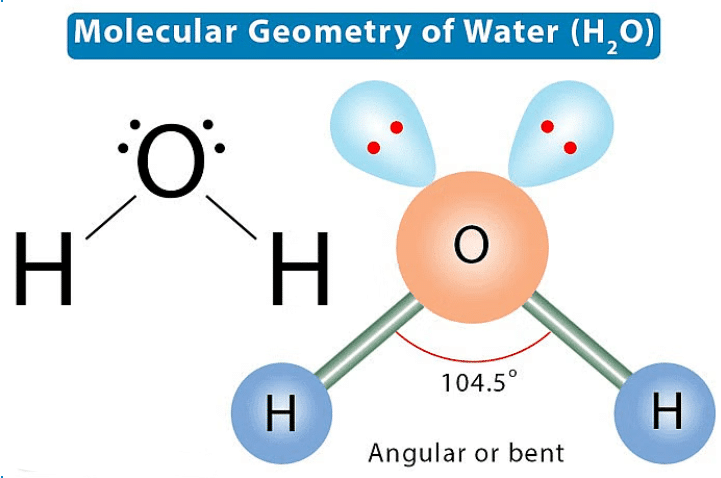 4. Electron Pair Geometry: This is the 3-D arrangement of electron pairs around the central atom of a polyatomic ion or molecule.
4. Electron Pair Geometry: This is the 3-D arrangement of electron pairs around the central atom of a polyatomic ion or molecule.